Official AQA AS Chemistry Unit 1 - 23Rd May 2013
Scroll to see replies
You'd use the carbon that's closest to the additional group. For example, instead of using '3-methylbutane' (as it might look), you'd use '2-methylbutane'.
This also applies to double bonds..
Instead of 'but-3-ene' it would be 'but-1-ene'
This also applies to double bonds..
Instead of 'but-3-ene' it would be 'but-1-ene'

(edited 10 years ago)
ah right, thanks both of you

This exam has come around so soon!
Those of you who did the Jan 2013 paper, how did you find it?
This will be my first and hopefully last sitting of the paper.
Those of you who did the Jan 2013 paper, how did you find it?

This will be my first and hopefully last sitting of the paper.
Original post by Octahedral
Take this for example
You would deduce which way you look at it gives you the smallest number on the carbon chain, from left to right it would be 4,4-DiChloro-3-MethylPentane. You then would look at it from the other way (right to left) and it would be 2,2-DiChloro-3-MethylPentane.
As you can see the position of the functional groups on the 2nd Haloalkane is lower than the 1st Haloalkane so that would be it.
Hope this makes sense.
Anybody help me with this?
i)Write an equation to show how NO is formed and give a condition needed for its formation[2]
ii) Deduce an equation to show how NO2 reacts with H20 & O2 to form HNO3[1]
You would deduce which way you look at it gives you the smallest number on the carbon chain, from left to right it would be 4,4-DiChloro-3-MethylPentane. You then would look at it from the other way (right to left) and it would be 2,2-DiChloro-3-MethylPentane.
As you can see the position of the functional groups on the 2nd Haloalkane is lower than the 1st Haloalkane so that would be it.
Hope this makes sense.
Anybody help me with this?
i)Write an equation to show how NO is formed and give a condition needed for its formation[2]
ii) Deduce an equation to show how NO2 reacts with H20 & O2 to form HNO3[1]
not sure about part i) but for part ii) 4NO2 +2H2O + O2 -> 4HNO3

Original post by Nav_Mallhi
This exam has come around so soon!
Those of you who did the Jan 2013 paper, how did you find it?
This will be my first and hopefully last sitting of the paper.
Those of you who did the Jan 2013 paper, how did you find it?

This will be my first and hopefully last sitting of the paper.
I found it really hard

Original post by Nav_Mallhi
This exam has come around so soon!
Those of you who did the Jan 2013 paper, how did you find it?
This will be my first and hopefully last sitting of the paper.
Those of you who did the Jan 2013 paper, how did you find it?

This will be my first and hopefully last sitting of the paper.
I done the Jan 2013 paper as a mock, it was quite easy tbh..not so great for us then :/
Jan 2013 paper and mark scheme for anyone who wants it
https://www.dropbox.com/sh/4m2j1fye6mpcvbz/PlhQvakB_z
https://www.dropbox.com/sh/4m2j1fye6mpcvbz/PlhQvakB_z
i get really confused with bond angles, i'm alright with drawing them out but when it asks you to predict the bond angle, I never know what it is :s help 

Original post by tumblrgirl
i get really confused with bond angles, i'm alright with drawing them out but when it asks you to predict the bond angle, I never know what it is :s help 

Just remember that the bond angle in a tetrahedral shape is 109.5 and each lone pair reduces the angle by 2.5 so for example a trigonal pyramidal shape has 3 bonded pairs and 1 lone pair so 109.5 - 2.5 = 107 which is the bond angle in the trigonal pyramidal shape. Hopefully that makes sense
Here is all of my Unit 1 notes. Most of it is pretty personalised and may have random things or tangents I went into. Also a lot is paraphrased from the book. 

Original post by SonamH
Just remember that the bond angle in a tetrahedral shape is 109.5 and each lone pair reduces the angle by 2.5 so for example a trigonal pyramidal shape has 3 bonded pairs and 1 lone pair so 109.5 - 2.5 = 107 which is the bond angle in the trigonal pyramidal shape. Hopefully that makes sense
thank you

Original post by tumblrgirl
not sure about part i) but for part ii) 4NO2 +2H2O + O2 -> 4HNO3 

for part i) you have Nitrogen and Oxygen in an engine so the reaction is 1/2 N2 + 1/2 O2 -> NO or you can have a whole number equation of N2 + O2 -> 2NO and you need a high temperature or a spark for this reaction to occur.
Does anyone else have problems with general calculations? D: Amounts of Substance? Any resource to help strengthen this area since I seem to be okay with the straightforward ones but as they add more steps I get extremely confused.
Also when they give you for example, and eqtn and they'd ask how much the concentration would be for 1 mole of blah blah substance D:
I wish I could elaborate further.
Any assistance please?
Also when they give you for example, and eqtn and they'd ask how much the concentration would be for 1 mole of blah blah substance D:
I wish I could elaborate further.
Any assistance please?
Original post by Nav_Mallhi
This exam has come around so soon!
Those of you who did the Jan 2013 paper, how did you find it?
This will be my first and hopefully last sitting of the paper.
Those of you who did the Jan 2013 paper, how did you find it?

This will be my first and hopefully last sitting of the paper.
I sat the Jan exam and got an A, so anyone, feel free to ask questions while you still have the chance, I'll be happy to help, just glad I don't have to sit it again
Original post by tumblrgirl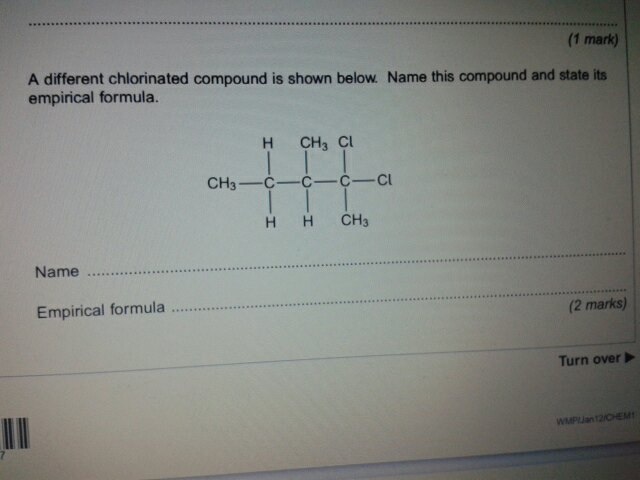
so I came across this question in Jan12 paper and I got a completely different answer from the markscheme on what the name of it is...
the markscheme says it's called 2,2 dichloro 3 methyl pentane....i don't understand why, anyone wanna help me?
so I came across this question in Jan12 paper and I got a completely different answer from the markscheme on what the name of it is...
the markscheme says it's called 2,2 dichloro 3 methyl pentane....i don't understand why, anyone wanna help me?

I always get super confused by these!!
I find it really helpful to fully draw out the displayed formula if you're stuck, it makes it easier to see methyl groups
Hello there everyone I want to say good luck to everyone epically to my friend umar and Ahmed and Minaan(snowman)
Hello there every one I want to say good luck to everyone epically my friend umar and Ahmed and Minaan(snowman)
Original post by mynameisntbobk
I sat the Jan exam and got an A, so anyone, feel free to ask questions while you still have the chance, I'll be happy to help, just glad I don't have to sit it again
You Beast!! .I found it extremely hard especially the last questions.There were alot of calculations on that paper ,it put me off.
I've got a question.When working out bond angles of ions when it's a positively charged ion does that mean the central atom has lost an electron so you don't include that electron in the shape .When its a negative charge an electron has been gained.ahh I'm so confused :/
Thanks in advance

Posted from TSR Mobile
Original post by mynameisntbobk
I sat the Jan exam and got an A, so anyone, feel free to ask questions while you still have the chance, I'll be happy to help, just glad I don't have to sit it again
That is very sweet of you.

And well done for the A!
Original post by chelley2
You Beast!! .I found it extremely hard especially the last questions.There were alot of calculations on that paper ,it put me off.
I've got a question.When working out bond angles of ions when it's a positively charged ion does that mean the central atom has lost an electron so you don't include that electron in the shape .When its a negative charge an electron has been gained.ahh I'm so confused :/
Thanks in advance
Posted from TSR Mobile
I've got a question.When working out bond angles of ions when it's a positively charged ion does that mean the central atom has lost an electron so you don't include that electron in the shape .When its a negative charge an electron has been gained.ahh I'm so confused :/
Thanks in advance

Posted from TSR Mobile
Haha thank you
 it was definitely a hard exam, so I was actually surprised I got the grade I did, I lost most of my marks in the calculations though
it was definitely a hard exam, so I was actually surprised I got the grade I did, I lost most of my marks in the calculations though  and a few in question 1 and 2
and a few in question 1 and 2And yep, you got the completely right, if its positive, the central atom has lost an electron, so its not included in the shape, vice versa for negative charge

Quick Reply
Related discussions
- A-level Exam Discussions 2024
- GCSE Exam Discussions 2024
- A Level Exam Discussions 2023
- GCSE Exam Discussions 2023
- Over 500 questions on AQA Bio Unit 4 + Current Spec and old Spec papers + MS!
- A-level Business Study Group 2022-2023
- Edexcel chemistry unit 2 mixed questions
- AQA A Level Law Paper 1 (7162/1) - 23rd May 2024 [Exam Chat]
- A-level Chemistry Study Group 2022-2023
- School is killing me - Y11 "GYG" 2022
- WJEC A Level History Paper 1 - 23rd May 2024 [Exam Chat]
- OCR AS-Level History Unit 2 (Y243,Y249,Y251-253) - 23rd May 2023 [Exam Chat]
- Self-teaching Chemistry A-level (as a private candidate)?
- SQA Exam Discussions 2024
- AQA Chem Unit 1 May 22nd 2015 *OFFICIAL THREAD*
- AQA A Level History Paper 1 (7042/1A-1L) - 23rd May 2024 [Exam Chat]
- WJEC A Level Business A2 Unit 3 (1510U30-1) - 23rd May 2023 [Exam Chat]
- UNIQ 2024 Participants Thread
- A-level History Study Group 2022-2023
- AQA A-level English Language Paper 1 (7702/1) - 23rd May 2024 [Exam Chat]
Latest
Trending
Last reply 1 day ago
OCR A-LEVEL CHEMISTRY A PAPER 2 (H432/02) - 21st June [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 2 (7405/2) - 18th June 2024 [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 1 (7405/1) - 10th June 2024 [Exam Chat]Last reply 1 week ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 2 weeks ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 Higher Tier Triple (8462 2H) - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 Foundation Triple (8462 1F) - 17th May 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 5 Chem 2 Foundation - 11th June 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]Trending
Last reply 1 day ago
OCR A-LEVEL CHEMISTRY A PAPER 2 (H432/02) - 21st June [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 2 (7405/2) - 18th June 2024 [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 1 (7405/1) - 10th June 2024 [Exam Chat]Last reply 1 week ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 2 weeks ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 Higher Tier Triple (8462 2H) - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 Foundation Triple (8462 1F) - 17th May 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 5 Chem 2 Foundation - 11th June 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]



