The mole concept questions
Original post by Sofya
Hello!
Please, can you explain to me how to solve this questions? I really don't understand!
Thank you in advance!
1) How many oxygen atoms are present in 1.5 moles of copper(II) sulfate pentahydrate, CuSO4·5H2O?
2)Which of the following contains the same number of ions as twice the value of Avogadro’s constant?
A)0.5 mol NaBr
B)1.0 mol Li2O
C)1.0 mol CaO
D)0.5 mol MgI2
3) How many individual ions does one mole of aluminium carbonate contain?
4)Silver oxide, Ag2O, can be decomposed to silver metal, Ag, plus oxygen gas, O2. How many molecules oxygen gas will form when 4.64 g of solid silver oxide is decomposed? The formula mass of silver oxide is 231.72 gmol-1.
5)How many moles of CO2 are produced when 1.0 mol of KMnO4, 5.0 mol of H2C2O4, and 3.0 mol of H2SO4 are mixed?
2KMnO4 + 5H2C2O4 + 3H2SO4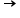 K2SO4 + 2MnSO4 + 10CO2 + 8H2O
K2SO4 + 2MnSO4 + 10CO2 + 8H2O
o0
Please, can you explain to me how to solve this questions? I really don't understand!
Thank you in advance!
1) How many oxygen atoms are present in 1.5 moles of copper(II) sulfate pentahydrate, CuSO4·5H2O?
2)Which of the following contains the same number of ions as twice the value of Avogadro’s constant?
A)0.5 mol NaBr
B)1.0 mol Li2O
C)1.0 mol CaO
D)0.5 mol MgI2
3) How many individual ions does one mole of aluminium carbonate contain?
4)Silver oxide, Ag2O, can be decomposed to silver metal, Ag, plus oxygen gas, O2. How many molecules oxygen gas will form when 4.64 g of solid silver oxide is decomposed? The formula mass of silver oxide is 231.72 gmol-1.
5)How many moles of CO2 are produced when 1.0 mol of KMnO4, 5.0 mol of H2C2O4, and 3.0 mol of H2SO4 are mixed?
2KMnO4 + 5H2C2O4 + 3H2SO4
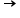 K2SO4 + 2MnSO4 + 10CO2 + 8H2O
K2SO4 + 2MnSO4 + 10CO2 + 8H2Oo0
The definitions of Avogadro's constant (number) are:
1 mole = 6.02 x 1023 particles of any substance.
The mass of 1 mol of any species has a mass equal to the relative mass expressed in grams.
1.
How many atoms of O in the formula?
How many atoms is in one mole?
How many moles do you have?
Multiply all above together for answer.
2.
How many ions in the formula?
How many moles of the compound?
How much is a mole?
Multiply the above together.
Is this number the same as twice Avogadro's constant?
3.
How many ions does aluminium carbonate contain?
The answer is that number times Avogadro's number.
4.
n=m/Mr
Look at the ratio Ag2O ---> 2Ag + 1/2 (O2)
5.
Which is the limiting number of moles of reactants?
What proportion do you have compared to what you need?
All of the product amounts will scale to that proportion.
How many atoms of O in the formula?
How many atoms is in one mole?
How many moles do you have?
Multiply all above together for answer.
2.
How many ions in the formula?
How many moles of the compound?
How much is a mole?
Multiply the above together.
Is this number the same as twice Avogadro's constant?
3.
How many ions does aluminium carbonate contain?
The answer is that number times Avogadro's number.
4.
n=m/Mr
Look at the ratio Ag2O ---> 2Ag + 1/2 (O2)
5.
Which is the limiting number of moles of reactants?
What proportion do you have compared to what you need?
All of the product amounts will scale to that proportion.
Original post by Infraspecies
n=m/Mr
Look at the ratio Ag2O ---> 2Ag + 1/2 (O2)
n=m/Mr
Look at the ratio Ag2O ---> 2Ag + 1/2 (O2)
I don't understand how to use formula mass in order to figure out molecules of oxygen ( Please, maybe you can write me how should I do this? I just can't understand it so far!!! Thank you very much
Original post by Sofya
I don't understand how to use formula mass in order to figure out molecules of oxygen ( Please, maybe you can write me how should I do this? I just can't understand it so far!!! Thank you very much
You have the mass of reactant.
Work out how many moles there are using the formula mass and the mass and the equation I gave you, which you should already know.
Look at the reaction ratio.
Scale the number of moles of product to oxygen gas.
Work out the moles of oxygen gas present.
Times this by Avogadro's number to work out the number of oxygen molecules.
Quick Reply
Related discussions
- chem question
- A level chemistry
- Molarity, etc.
- A level physics nuclear question
- Help with a question: equilibria
- Is Chemistry better at A-level than at GCSE?
- Volume
- chemistry aqa a-level question help
- Drift Velocity Q
- Chemistry moles help
- Mole calculation
- Chem alevel help
- AS chemistry
- a level chem help
- Titrations
- Kc Titration Question
- Aspirin
- AQA Chemistry Chapter 2 Practice Questions HELP
- Chemistry A Level Titrations Help
- hard titration alevel chem Q!
Latest
Trending
Last reply 1 week ago
Im confused about this chemistry question, why does it form these productsTrending
Last reply 1 week ago
Im confused about this chemistry question, why does it form these products



