Boltzmann Distribution - question
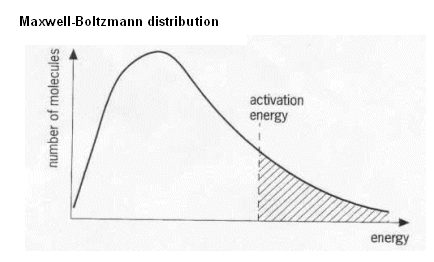
There is a single statement in my textbook that I am trying to get my head around and having issues with.
The book states "there is no maximum energy for a molecule - the curve does not meet the x-axis at high energy. The curve would need to reach infinite energy to meet the x-axis"
The highlighted part implies that reaching the x-axis would imply reaching infinite energy. Why is that so?
My understanding of this is that:

As shown on my sketch - the line reaching the x-axis should only imply the presence of molecules getting to a certain level of energy and no more being able to do so. Why does the book state otherwise?
And sorry, I know this is just a theoretical question, but I am really picky on those little bits that don't add up and leave holes in my understanding.
frostyy
The Maxwell-Boltzmann distribution is defined such that the function has an asymptote at x-axis=0, i.e. the function will only touch the x-axis in the limit at infinity:
The probability density function (what you drew) is proportional to where is a function of T.
The distribution is just a function which models the system, so this is perfectly fine. In reality for an isolated system an individual particle can never have more energy than the total energy of the system but the distribution is still a very good approximation to reality.
It's also worth noting there is a difference between the Boltzmann distribution and the Maxwell-Boltzmann distribution:
https://www.wikiwand.com/en/Boltzmann_distribution
https://www.wikiwand.com/en/Maxwell%E2%80%93Boltzmann_distribution
The probability density function (what you drew) is proportional to where is a function of T.
The distribution is just a function which models the system, so this is perfectly fine. In reality for an isolated system an individual particle can never have more energy than the total energy of the system but the distribution is still a very good approximation to reality.
It's also worth noting there is a difference between the Boltzmann distribution and the Maxwell-Boltzmann distribution:
https://www.wikiwand.com/en/Boltzmann_distribution
https://www.wikiwand.com/en/Maxwell%E2%80%93Boltzmann_distribution
(edited 7 years ago)
Quick Reply
Related discussions
- Maxwell-Boltzmann distribution curve help
- Thermodynamics CMB would to approach question
- Probability help
- Hypothesis Testing
- Binomial hypothesis test question
- HNC Maths Question Hand Drawn Sketch
- Probability distribution
- Binomial distribution
- Cie as level statistics question , please help
- Maths help
- probability and statistics 2 , poisson distribution
- Negative Binomial Question
- HELPPP A level maths linear interpolation
- Discrete probability disturbution
- Negative Binomial Question
- Anyone know where to find the 2023 AS Edexcel Further Maths papers?
- cumalative negative binomial
- Central Limit Theorem: How do you calculate the test statistic?
- Wjec a level unit 4 maths
- Urgent-stats al maths q!
Latest
Trending
Last reply 1 week ago
Im confused about this chemistry question, why does it form these productsTrending
Last reply 1 week ago
Im confused about this chemistry question, why does it form these products



