Original post by epicnm
Are hydrogenation of Alkenes (to form alkanes) and hydration of alkenes (to form alcohols) electrophilic addition reactions.
I’ve seen different things about this online and the OCR spec groups all of the Alkenes reactions as ‘addition reactions’.
I’ve seen different things about this online and the OCR spec groups all of the Alkenes reactions as ‘addition reactions’.
Yes these would be, it’s addition as you are only adding atoms to the alkene, not removing any to do this, and it’s electrophilic as they are electron pair acceptors.
Any reaction that involves one thing reacting with an alkene breaking the double bond and forming one product is an addition reaction.
C2H4 + H2 --> C2H6 is definitely addition
C2H4 + H2O --> C2H5OH is addition
C2H4 + Br2 --> C2H4Br2 is addition
C2H4 + HBr --> C2H5Br is addition
C2H4 + H2 --> C2H6 is definitely addition
C2H4 + H2O --> C2H5OH is addition
C2H4 + Br2 --> C2H4Br2 is addition
C2H4 + HBr --> C2H5Br is addition
Original post by Wombatsalamander
Yes these would be, it’s addition as you are only adding atoms to the alkene, not removing any to do this, and it’s electrophilic as they are electron pair acceptors.
Original post by Davies Chemistry
Any reaction that involves one thing reacting with an alkene breaking the double bond and forming one product is an addition reaction.
C2H4 + H2 --> C2H6 is definitely addition
C2H4 + H2O --> C2H5OH is addition
C2H4 + Br2 --> C2H4Br2 is addition
C2H4 + HBr --> C2H5Br is addition
C2H4 + H2 --> C2H6 is definitely addition
C2H4 + H2O --> C2H5OH is addition
C2H4 + Br2 --> C2H4Br2 is addition
C2H4 + HBr --> C2H5Br is addition
Ahhh that makes a lot more sense! Thank you!
The hydrogenation reaction of alkenes to alkanes is not electrophilic addition. In hydrogenation, a nickel catalyst and hydrogen gas is used (and no electrophile is involved), and there is a different mechanism taking place. This mechanism involves adsorption of the alkene to the surface of the nickel catalyst, so it weakly forms bonds with the nickel catalyst which allows hydrogen to be added to the molecule. It is still an addition reaction, but is not electrophilic addition.
In terms of hydration, there is electrophilic addition involved.
In step one, a proton from the phosphoric acid catalyst acts as the electrophile and accepts a lone pair from the C=C double bond, forming a carbocation intermediate.
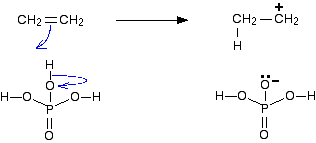
This site has a great explanation of the full mechanism: https://www.chemguide.co.uk/physical/catalysis/hydrate.html
Hope this helps, and anyone please correct me if there are any mistakes
In terms of hydration, there is electrophilic addition involved.
In step one, a proton from the phosphoric acid catalyst acts as the electrophile and accepts a lone pair from the C=C double bond, forming a carbocation intermediate.

This site has a great explanation of the full mechanism: https://www.chemguide.co.uk/physical/catalysis/hydrate.html
Hope this helps, and anyone please correct me if there are any mistakes

Quick Reply
Related discussions
- Electrophillic/nucelophillic substitution /addition
- Nucelophiles Vs Ligands
- electrophilic addition reactions with sulphuric acid? Please help!
- Chemistry - Benzene
- Does it matter where the chlorine is in vinyl chloride?
- Skeletal Formulae
- Chemistry help on benzene
- AQA A Level Chemistry
- AS chemistry paper 2 2022 AQ
- Alevel Chemistry Aromatic Compounds
- Organic Synthesis help
- AlCl3 reacting with benzene
- Random OCR A-level organic mechanism reactions paper 3
- AQA A-Level Chemistry Paper 2 (7405/2) - 19th June 2023 [Exam Chat]
- AQA A Level Chemistry Paper 3 7405/3 - 23 Jun 2022 [Exam Chat]
- additional maths content in A level maths textbooks?
- Gcse additional paper
- PAG 4.1 identifying Unknowns OCR A chemistry
- Learn lots of maths fast
- A-level further maths without IGCSE additional maths?
Latest
Trending
Last reply 1 week ago
Im confused about this chemistry question, why does it form these productsTrending
Last reply 1 week ago
Im confused about this chemistry question, why does it form these products



