Chemistry - pi bonds
I am finding it really hard to visualise a pi orbital in alkenes
I understand the p orbitals overlap to form a pi orbital which forms a cloud of electrons above and below the bond but I am finding it hard to visualise?
Could someone please explain this to me in terms of the overlapping of the p orbitals and also how S-orbitals come about?
thanks
I understand the p orbitals overlap to form a pi orbital which forms a cloud of electrons above and below the bond but I am finding it hard to visualise?
Could someone please explain this to me in terms of the overlapping of the p orbitals and also how S-orbitals come about?
thanks
I think you're justified in finding it hard to visualise. It's not really until 1st year chemistry where you learn about molecular orbital theory which makes this much easier to understand.
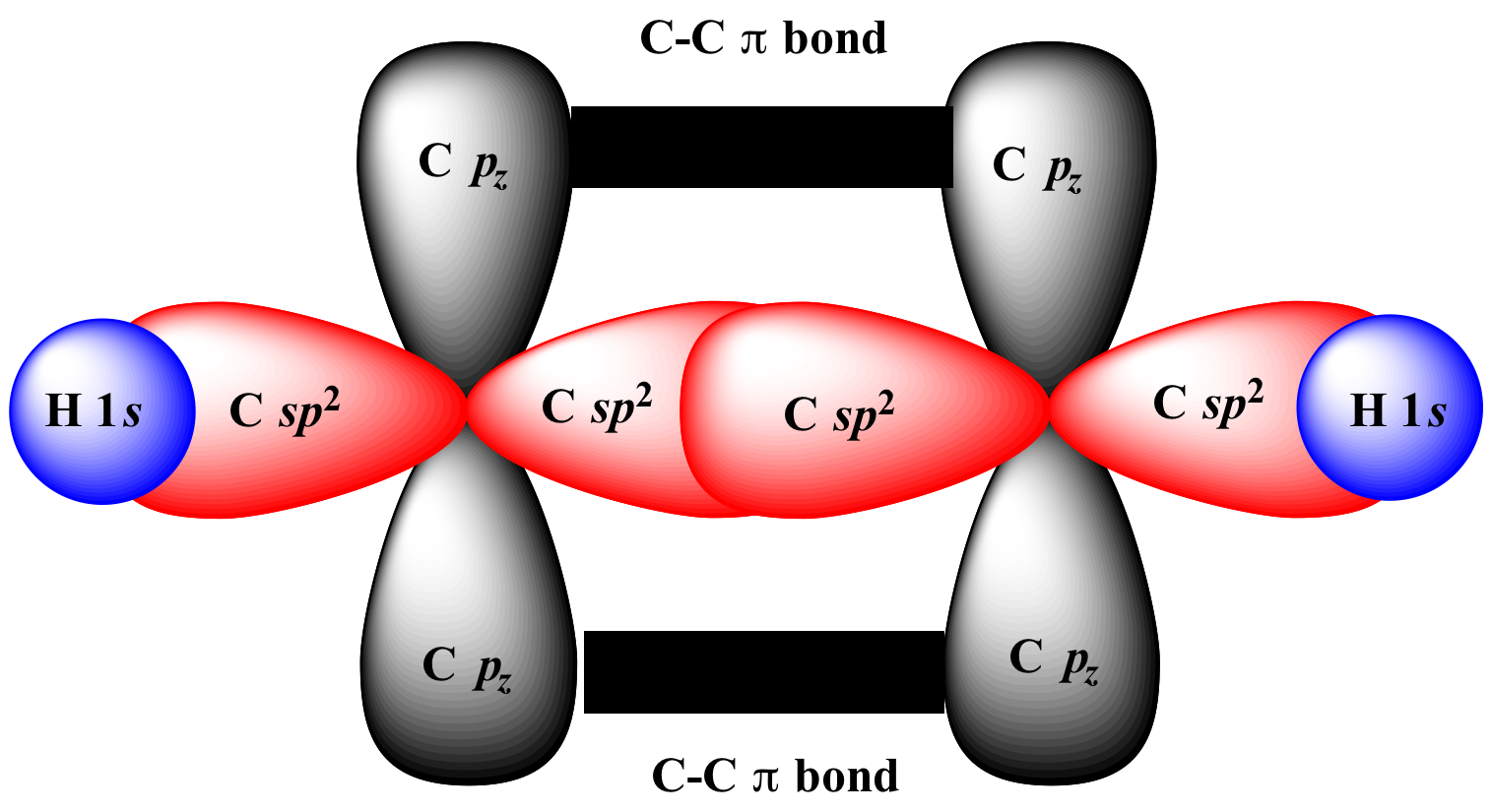
I'll try to be as basic as possible!
The carbons here, are sp2 hybridised.
This means that each of the carbons 4 valence electrons (2s2, 2p2) have combined into 3 sp2 hybridised orbitals, and one unhybridised p-orbital.
Basically, the pi bond forms because the unhybridised p-orbitals of each sp2 hybridised carbon align above and below the plane of the molecule.
And now the super simple version!
Sigma bonds are formed by 'end on' overlap, I've highlighted in red (Like they're kissing)
Pi bonds are formed by 'edge on' overlap, highlighted in black. (They side-by-side rather than kissing)
Hope this helps!

I'll try to be as basic as possible!
The carbons here, are sp2 hybridised.
This means that each of the carbons 4 valence electrons (2s2, 2p2) have combined into 3 sp2 hybridised orbitals, and one unhybridised p-orbital.
Basically, the pi bond forms because the unhybridised p-orbitals of each sp2 hybridised carbon align above and below the plane of the molecule.
And now the super simple version!
Sigma bonds are formed by 'end on' overlap, I've highlighted in red (Like they're kissing)
Pi bonds are formed by 'edge on' overlap, highlighted in black. (They side-by-side rather than kissing)
Hope this helps!
(edited 3 months ago)
Quick Reply
Related discussions
- organic chemistry:Hybridization
- Chemistry help on benzene
- Dissociation chemistry
- Ask Us Anything!
- Lewis structure for SO4 2-
- EZ isomerism
- Could someone please help with a mechansim question.
- AS/A Level Chemistry Study Group 2023/2024
- Chem alevel help
- Remembering Exothermic and Endothermic
- Hydrolysis
- chemistry question
- A Level Chemistry HELP
- bio help a level
- van der waals
- Ionic and covalent bonding
- OCR Biology Help:
- A-level chemistry. Draw a labelled diagram to show the formation of the π-bond.
- A Level Chemistry Ligand Question
- chemistry help
Latest
Trending
Last reply 1 week ago
Im confused about this chemistry question, why does it form these productsTrending
Last reply 1 week ago
Im confused about this chemistry question, why does it form these products



