OCR Chemistry A Exam Thread (Breadth - May 27 2016 and Depth - June 10 2016)
Scroll to see replies
Original post by Clintbarton
how can ya'll remember what you got for maths questions? I forget as soon as i come out!
Saaame 😫😂😂
Original post by Nikosunway
For the question on polarity, I am sure it is C, due to the fact that if you draw the molecule , it is symmetrical, thus poles repel.
Ah but when you drew the one with the E isomer with two chlorines either side of the C-C double bond then they should cancel out also??
Original post by 87Mack
nah why do you square it? there was only one mole of SO3
There were 2 moles
2SO2 + O2 -> 2SO3
I'm supposed to be in chemistry right now lol do they think i'm going to lesson after the exam so ppl can tell me I got everything wrong BYEEE
Original post by JohnMcButts
There were 2 moles
2SO2 + O2 -> 2SO3
2SO2 + O2 -> 2SO3
Woopsies I read it as 3
It was an reasonable paper, but I know I've made some silly mistakes already... There were some multiple choice questions were really trying to trick you.
Posted from TSR Mobile
Posted from TSR Mobile
Original post by JohnMcButts
There were 2 moles
2SO2 + O2 -> 2SO3
2SO2 + O2 -> 2SO3
it only had 1 mole in the equation given tho
Original post by Barklimus
Pretty that the reagent for turning the the alkene into the blank product (C4H8Br2) was Br2. Then, to turn that into a diol, you could use NaOH.
I put that too but Cl2 instead of Br2!
Original post by A Sajid
Low temperatures push the reaction to the exothermic side. And the forward reaction/ product had less molecules than the reactants. So increase in pressure pushed the equilibrium to the product side. And then you talk about how high pressure costly blah blah.
But if low temp and high pressure push the equilibrium to the product/right hand side then surely that wouldn't produce a good percentage yield since more of the product would break down to form reactants?? There was a similar question in one of the specimen depth papers which is had a contradictory answer to the question in today's paper?
Original post by Jitesh
Certainly but not sure how many with it being a new spec and all
Ahh ok that's alright then, hopefully won't lose too many marks
Original post by Studentet
I put that too but Cl2 instead of Br2!
Same thing because they're both halogenation?
Original post by Barklimus
AHCK You needed to square root it, cos that value is (SO3) squared. I got about 0.876moldm-3.
Yep that's what I got as well
Original post by Studentet
I put that too but Cl2 instead of Br2!
That's fine it would be alright for any suitable reagent
Br2 would just show a clearer colour change and is normally used in experiments so it would probably be more common for people to put that
Original post by Jitesh
It's a bit blurry but the top circle on the Oxygen I drew as a different symbol but I was not sure if it meant different as in a circle when uses crosses to represent it??
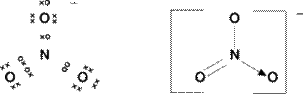

I used it for the dative one. What did they mean?
Original post by A Sajid
Lmao what kind of science has this don been doing. Its like saying you have to cube 02 

im a don now is it? nah but seriously tho wasnt it only one mole ?? if its not i got that wrong then.
Original post by snickercell
Did they?? Dang it! Would i still get some marks for correct working out albeit with wrong numbers?
I'm not sure as that was the first step. You might get 1 for method
Original post by 87Mack
concentration of S03 = 0.768 anyone????????
I think I got something like that.
Original post by Barklimus
AHCK You needed to square root it, cos that value is (SO3) squared. I got about 0.876moldm-3.
I got this
Original post by 87Mack
nah why do you square it? there was only one mole of SO3
You have to look at the stoichiometry
Original post by Clintbarton
That one messed me up and I ran out of time at the end to finish working out, I think i put A because B (or C) wasn't even E/Z.
They were all E/Z, even the ones with the chloros not on the double bonding carbons had hydrogen and methyls on the double bonding carbons (so two different atoms/groups of atoms)
Original post by tamoni4
Yes, exactly. Since there were 2 measurment, you had to double the error and then divide by the mass of water and multiply by 100.
Lol I forgot to double the %error, gg me
... I really don't know whether to be happy that the exam was relatively decent or scared by the fact that this will usually mean a high grade boundary.
Btw, do you guys know what reagent was used to add that oxygen in the second stage of prep in that last question?
Posted from TSR Mobile
Btw, do you guys know what reagent was used to add that oxygen in the second stage of prep in that last question?
Posted from TSR Mobile
Quick Reply
Related discussions
- A-level Exam Discussions 2024
- OCR A GCSE Chemistry Paper 1 (Foundation Combined) J250/03- 22nd May 2023 [Exam Chat]
- OCR A GCSE Chemistry Paper 1 (Higher Combined) J250/09- 22nd May 2023 [Exam Chat]
- OCR A GCSE Chemistry Paper 2 (Foundation Combined) J250/04- 13th June [Exam Chat]
- Grade Growth Chronicles | From C's to A's (23-24)
- GCSE Biology Study Group 2022-2023
- OCR A GCSE Physics Paper 1 (Foundation Combined) J250/05- 25th May 2023 [Exam Chat]
- GCSE Exam Discussions 2023
- OCR A GCSE Physics Paper 2 (Foundation Combined) J250/06- 16th June 2023 [Exam Chat]
- OCR A GCSE Physics Paper 2 (Higher Combined) J250/12- 16th June 2023 [Exam Chat]
- OCR A GCSE Biology Paper 2 (Higher Combined) J250/08 - 9th June 2023 [Exam Chat]
- GCSE Exam Discussions 2024
- OCR A GCSE Biology Paper 2 (Foundation Combined) J250/02- 9th June 2023 [Exam Chat]
- OCR A GCSE Physics Paper 1 (Higher Combined) J250/11- 25th May 2023 [Exam Chat]
- A Level Exam Discussions 2023
- OCR A GCSE Biology Paper 1 (Foundation Combined) J250/01- 16th May 2023 [Exam Chat]
- OCR A GCSE Biology Paper 1 (Higher Combined) J250/07- 16th May 2023 [Exam Chat]
- GCSE Chemistry Study Group 2023/2024
- OCR GCSE Geography A Paper 1 (J383/01) - 22nd May 2023 [Exam Chat]
- I achieved 45 Distinctions in an Access course with the DistanceLearningCentre. AMA!
Latest
Trending
Last reply 2 days ago
OCR A-LEVEL CHEMISTRY A PAPER 2 (H432/02) - 21st June [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 2 (7405/2) - 18th June 2024 [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 1 (7405/1) - 10th June 2024 [Exam Chat]Last reply 2 weeks ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 2 weeks ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 Higher Tier Triple (8462 2H) - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 Foundation Triple (8462 1F) - 17th May 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 5 Chem 2 Foundation - 11th June 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]Trending
Last reply 2 days ago
OCR A-LEVEL CHEMISTRY A PAPER 2 (H432/02) - 21st June [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 2 (7405/2) - 18th June 2024 [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 1 (7405/1) - 10th June 2024 [Exam Chat]Last reply 2 weeks ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 2 weeks ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 Higher Tier Triple (8462 2H) - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 Foundation Triple (8462 1F) - 17th May 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 5 Chem 2 Foundation - 11th June 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]



