Chemistry Bonding Question help?
3.Which type of bond is formed between N and B when a molecule of NH3 reacts with a molecule of BF3?
A Ionic.
B Covalent.
C Co-ordinate.
D Van der Waals.
Q5.Which of these substances does not show hydrogen bonding?
A HF
B NH3
C CH3COOH
D CHF3
.Which of these substances has permanent dipole-dipole attractions between molecules?
A CCl4
B C2F4
C (CH3)2CO
D CO2
Q9.In which one of the following species is the shape influenced by the presence of one or more lone pairs of electrons?
A NH
B NH
C [CH3NH3]+
D [Co(NH3)6]2+
I've attempted these questions but I just have no idea on how to get the correct answer. Could anyone explain how to get to the answer?
A Ionic.
B Covalent.
C Co-ordinate.
D Van der Waals.
Q5.Which of these substances does not show hydrogen bonding?
A HF
B NH3
C CH3COOH
D CHF3
.Which of these substances has permanent dipole-dipole attractions between molecules?
A CCl4
B C2F4
C (CH3)2CO
D CO2
Q9.In which one of the following species is the shape influenced by the presence of one or more lone pairs of electrons?
A NH
B NH
C [CH3NH3]+
D [Co(NH3)6]2+
I've attempted these questions but I just have no idea on how to get the correct answer. Could anyone explain how to get to the answer?
anyone
Original post by Bertybassett
anyone
Well I am not very good at chemistry (despite what my name may imply, as I am only in year 9) but what have you done so far?
TBH I only know a little about ionic and covalent bonds.
But someone else will come here and help, but show them what you have done so far, or what you think the answers are.
Which type of bond is formed between N and B when a molecule of NH3 reacts with a molecule of BF3?
A Ionic.
B Covalent. X Nitrogen and boron. n has lone pair, donates it to b
C Co-ordinate.
D Van der Waals.
Q5.Which of these substances does not show hydrogen bonding?
A HF
B NH3
C CH3COOH
D CHF3 X h is not bonded to F N or O
.Which of these substances has permanent dipole-dipole attractions between molecules?
A CCl4 (can't be this, its tetrahedral)
B C2F4 (planar molecule)
C (CH3)2CO
D CO2 X not sure but this is the only one that has a neg point and pos point of its 3d structure.
Q9.In which one of the following species is the shape influenced by the presence of one or more lone pairs of electrons?
A NH ??? if you meant nh3, that would be the answer
B NH ???? I'm confused, a and b- these are the same
C [CH3NH3]+
D [Co(NH3)6]2+
A Ionic.
B Covalent. X Nitrogen and boron. n has lone pair, donates it to b
C Co-ordinate.
D Van der Waals.
Q5.Which of these substances does not show hydrogen bonding?
A HF
B NH3
C CH3COOH
D CHF3 X h is not bonded to F N or O
.Which of these substances has permanent dipole-dipole attractions between molecules?
A CCl4 (can't be this, its tetrahedral)
B C2F4 (planar molecule)
C (CH3)2CO
D CO2 X not sure but this is the only one that has a neg point and pos point of its 3d structure.
Q9.In which one of the following species is the shape influenced by the presence of one or more lone pairs of electrons?
A NH ??? if you meant nh3, that would be the answer
B NH ???? I'm confused, a and b- these are the same
C [CH3NH3]+
D [Co(NH3)6]2+
(edited 6 years ago)
Original post by ionicbond
Which type of bond is formed between N and B when a molecule of NH3 reacts with a molecule of BF3?
A Ionic.
B Covalent. X Nitrogen and boron. n has lone pair, donates it to b
C Co-ordinate.
D Van der Waals.
Q5.Which of these substances does not show hydrogen bonding?
A HF
B NH3
C CH3COOH
D CHF3 X h is not bonded to F N or O
.Which of these substances has permanent dipole-dipole attractions between molecules?
A CCl4 (can't be this, its tetrahedral)
B C2F4 (planar molecule)
C (CH3)2CO
D CO2 X not sure but this is the only one that has a neg point and pos point of its 3d structure.
Q9.In which one of the following species is the shape influenced by the presence of one or more lone pairs of electrons?
A NH ???
B NH ???? I'm confused, a and b- these are the same
C [CH3NH3]+
D [Co(NH3)6]2+
A Ionic.
B Covalent. X Nitrogen and boron. n has lone pair, donates it to b
C Co-ordinate.
D Van der Waals.
Q5.Which of these substances does not show hydrogen bonding?
A HF
B NH3
C CH3COOH
D CHF3 X h is not bonded to F N or O
.Which of these substances has permanent dipole-dipole attractions between molecules?
A CCl4 (can't be this, its tetrahedral)
B C2F4 (planar molecule)
C (CH3)2CO
D CO2 X not sure but this is the only one that has a neg point and pos point of its 3d structure.
Q9.In which one of the following species is the shape influenced by the presence of one or more lone pairs of electrons?
A NH ???
B NH ???? I'm confused, a and b- these are the same
C [CH3NH3]+
D [Co(NH3)6]2+
You have a very fitting name for this...
Original post by Bill Nye
You have a very fitting name for this...
hahah, i suppose i do. not 100% sure about the answers but as an A2 chemist, its my best shot
Original post by ionicbond
Which type of bond is formed between N and B when a molecule of NH3 reacts with a molecule of BF3?
A Ionic.
B Covalent. X Nitrogen and boron. n has lone pair, donates it to b
C Co-ordinate.
D Van der Waals.
Q5.Which of these substances does not show hydrogen bonding?
A HF
B NH3
C CH3COOH
D CHF3 X h is not bonded to F N or O
.Which of these substances has permanent dipole-dipole attractions between molecules?
A CCl4 (can't be this, its tetrahedral)
B C2F4 (planar molecule)
C (CH3)2CO
D CO2 X not sure but this is the only one that has a neg point and pos point of its 3d structure.
Q9.In which one of the following species is the shape influenced by the presence of one or more lone pairs of electrons?
A NH ??? if you meant nh3, that would be the answer
B NH ???? I'm confused, a and b- these are the same
C [CH3NH3]+
D [Co(NH3)6]2+
A Ionic.
B Covalent. X Nitrogen and boron. n has lone pair, donates it to b
C Co-ordinate.
D Van der Waals.
Q5.Which of these substances does not show hydrogen bonding?
A HF
B NH3
C CH3COOH
D CHF3 X h is not bonded to F N or O
.Which of these substances has permanent dipole-dipole attractions between molecules?
A CCl4 (can't be this, its tetrahedral)
B C2F4 (planar molecule)
C (CH3)2CO
D CO2 X not sure but this is the only one that has a neg point and pos point of its 3d structure.
Q9.In which one of the following species is the shape influenced by the presence of one or more lone pairs of electrons?
A NH ??? if you meant nh3, that would be the answer
B NH ???? I'm confused, a and b- these are the same
C [CH3NH3]+
D [Co(NH3)6]2+
for q9 it's nh2- and nh4-
also the markscheme states that the first question's answer is C, not B
(edited 6 years ago)
Original post by Bertybassett
for q9 it's nh2- and nh4-
also the markscheme states that the first question's answer is C, not B
also the markscheme states that the first question's answer is C, not B
Sorry thats what i meant, if n donates a lone PAIR it would be coordinate bonding anyway because theres no sharing from the b. i just put it next to the wrong letter.
Original post by ionicbond
Sorry thats what i meant, if n donates a lone PAIR it would be coordinate bonding anyway because theres no sharing from the b. i just put it next to the wrong letter.
thank you for the help. i was wondering if i'm struggling with these questions because i haven't actually learnt about 3d or linear shapes of molecules or anything? could this be so?
Original post by Bertybassett
thank you for the help. i was wondering if i'm struggling with these questions because i haven't actually learnt about 3d or linear shapes of molecules or anything? could this be so?
yes definitely, you need that knowledge for most of the questions. maybe you will learn it later in the year?
Original post by ionicbond
yes definitely, you need that knowledge for most of the questions. maybe you will learn it later in the year?
ah right thank you for the help. much appreciated.
3.Which type of bond is formed between N and B when a molecule of NH3 reacts with a molecule of BF3?
C Co-ordinate. ----there is donation of e pair from N to B
Q5.Which of these substances does not show hydrogen bonding?
D CHF3 -----------H is not bonded to very electronegative atom.
.Which of these substances has permanent dipole-dipole attractions between molecules?
C (CH3)2CO
Q9.In which one of the following species is the shape influenced by the presence of one or more lone pairs of electrons?
Answers are not clear.
C Co-ordinate. ----there is donation of e pair from N to B
Q5.Which of these substances does not show hydrogen bonding?
D CHF3 -----------H is not bonded to very electronegative atom.
.Which of these substances has permanent dipole-dipole attractions between molecules?
C (CH3)2CO
Q9.In which one of the following species is the shape influenced by the presence of one or more lone pairs of electrons?
Answers are not clear.
Original post by adianadiadi
3.Which type of bond is formed between N and B when a molecule of NH3 reacts with a molecule of BF3?
C Co-ordinate. ----there is donation of e pair from N to B
Q5.Which of these substances does not show hydrogen bonding?
D CHF3 -----------H is not bonded to very electronegative atom.
.Which of these substances has permanent dipole-dipole attractions between molecules?
C (CH3)2CO
Q9.In which one of the following species is the shape influenced by the presence of one or more lone pairs of electrons?
Answers are not clear.
C Co-ordinate. ----there is donation of e pair from N to B
Q5.Which of these substances does not show hydrogen bonding?
D CHF3 -----------H is not bonded to very electronegative atom.
.Which of these substances has permanent dipole-dipole attractions between molecules?
C (CH3)2CO
Q9.In which one of the following species is the shape influenced by the presence of one or more lone pairs of electrons?
Answers are not clear.
thanks. for 5 where the answer is d could you explain how you got to this answer as in how you know h isnt bonded to a very electronegative atom?
Original post by Bertybassett
thanks. for 5 where the answer is d could you explain how you got to this answer as in how you know h isnt bonded to a very electronegative atom?
could you please tell me
Original post by Bertybassett
could you please tell me
please
Original post by Bertybassett
thanks. for 5 where the answer is d could you explain how you got to this answer as in how you know h isnt bonded to a very electronegative atom?
It's because this is a substituted alkane, all haloalkanes are.
The central carbon atom has individual bonds to each of the other atoms, and the other atoms do not have any bonds to each other.
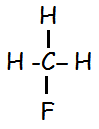
Original post by TutorsChemistry
It's because this is a substituted alkane, all haloalkanes are.
The central carbon atom has individual bonds to each of the other atoms, and the other atoms do not have any bonds to each other.
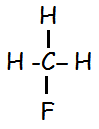
The central carbon atom has individual bonds to each of the other atoms, and the other atoms do not have any bonds to each other.
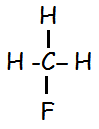
ah right thanks. i dont think i have learnt this yet in class so do you think i will because there's no way i'd know to do that structure for that formula in an exam at this moment in time?
Original post by Bertybassett
thanks. for 5 where the answer is d could you explain how you got to this answer as in how you know h isnt bonded to a very electronegative atom?
It is based on its structure. Look at the valency of C. It is 4. Hence it will form 4 bonds. One with H and three with F. Also note that, valencies of H and F are 1 and 1. They can form only one bond.
Quick Reply
Related discussions
- AS/A Level Chemistry Study Group 2023/2024
- Ask Us Anything!
- Dissociation chemistry
- chemistry question
- Hydrolysis
- A Level Chemistry Ligand Question
- A Level Chemistry HELP
- Random OCR A-level organic mechanism reactions paper 3
- A-level chemistry. Draw a labelled diagram to show the formation of the π-bond.
- Chemistry question organic
- Chemistry Olympiad question
- GCSE Bonding + Structure Question
- How do you do NMR questions!?
- chemistry help
- gcse macromolecules
- Does anyone why the answer to this a level chemistry question is A
- OCR Biology Help:
- organic chemistry:Hybridization
- Enthalpy change
- year 12 study journal!
Latest
Trending
Last reply 1 week ago
Im confused about this chemistry question, why does it form these productsTrending
Last reply 1 week ago
Im confused about this chemistry question, why does it form these products



