Equilibria
Why do we omit solids and liquids in the equilibrium expression? I know it has something to do with concentrations, but I don't get it.
And for homogeneous equilibrium, why are the liquids and solids included in the equilibrium constant, when they aren't in heterogeneous equilibrium?
And for homogeneous equilibrium, why are the liquids and solids included in the equilibrium constant, when they aren't in heterogeneous equilibrium?
(edited 6 years ago)
solids and liquids are constants as their concentrations don't change through out the reaction
homogenous equilibria they aren't included in homogenous either
homogenous equilibria they aren't included in homogenous either

Original post by SAMHANLEY88
solids and liquids are constants as their concentrations don't change through out the reaction
homogenous equilibria they aren't included in homogenous either
homogenous equilibria they aren't included in homogenous either

But what about this?
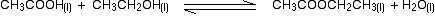
It was included in both my textbook and https://www.chemguide.co.uk/physical/equilibria/kc.html
(edited 6 years ago)
Original post by Mme_Bonii
But what about this?
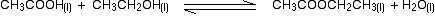
It was included in both my textbook and https://www.chemguide.co.uk/physical/equilibria/kc.html
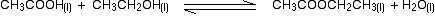
It was included in both my textbook and https://www.chemguide.co.uk/physical/equilibria/kc.html
ok I was taught by teachers otherwise so I'm gonna assume it's because if they're all liquids they're all in a mixture so their concentration is a fraction of the mixture so when the reactants are used up then the concentration in relation to other reactants and products changes (I assume)
Original post by Mme_Bonii
But what about this?
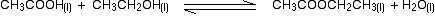
It was included in both my textbook and https://www.chemguide.co.uk/physical/equilibria/kc.html
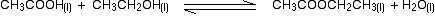
It was included in both my textbook and https://www.chemguide.co.uk/physical/equilibria/kc.html
i didn't explain that well
the reason liquids and solids are emitted when there are aqueous or gases is because their change is negligible
whereas compared to eachother they become significant
Original post by SAMHANLEY88
i didn't explain that well
the reason liquids and solids are emitted when there are aqueous or gases is because their change is negligible
whereas compared to eachother they become significant
the reason liquids and solids are emitted when there are aqueous or gases is because their change is negligible
whereas compared to eachother they become significant
Thank you so much!
Quick Reply
Related discussions
- chemistry as level equilibria help
- Hard friction question
- Ocr a level chem
- Game Theory question - URGENT HELP NEEDED!
- Ocr gateway a chemistry as level paper 2021 paper 2
- AQA Environmental science paper 2
- Equilibria and entropy changes at boiling temperature
- What affects Kp?
- Help with a question: equilibria
- Electrode potentials and equilibria?
- Biology and chemistry
- Chemical engineering or pharmacy
- Increase Predicted Grades ASAP Help
- My attempt at not sabotaging my future career!
- Grade Growth Chronicles | From C's to A's (23-24)
- AS Chemistry Paper 1 2022
- Edexcel IGCSE Biology 9-1 | PAPER 1
- word ending game
- AS/A Level Chemistry Study Group 2023/2024
- Revision Struggles?! Join the 2023 TSR All Day Revision Thread!
Latest
Trending
Last reply 1 week ago
Im confused about this chemistry question, why does it form these productsTrending
Last reply 1 week ago
Im confused about this chemistry question, why does it form these products



