This discussion is now closed.
Check out other Related discussions
- A-level Exam Discussions 2024
- GCSE Exam Discussions 2024
- ⭐niamhcheesecake's Yr 11 journal: help me conquer Gcse's :(( ⭐
- GCSE Diary
- School is killing me - Y11 "GYG" 2022
- IAL repeats cash in.
- GCSE Exam Discussions 2023
- Edexcel chemistry unit 2 mixed questions
- A Level Advice
- A Level Exam Discussions 2023
- Who will win the EFL Cup?
- Edexcel Past Papers
- Synoptic questions
- 4 weeks Until 1st Year Med Exams
- Entry requirements university of Hertfordshire
- a level chemistry
- Higher chemistry
- Chemistry 1C june 2023 exam 22nd May
- Bangor University GCSE Revision guides?
- AS/A Level Chemistry Study Group 2023/2024
Edexcel Chemistry Unit 1 (23/05/2013)
Scroll to see replies
Original post by jemma01
Does anybody know what diagrams of experiments we need to know? - the ones that we have to draw
We need to know how to draw the apparatus for:
Fractional distillation
simples distillation
Heat to reflux
Collecting a gas over water and in a gas syringe (for measuring rates of reaction).
Specific experiments? The electrolysis of copper chromate was on a past paper. Then there was also the determining of the enthalpy change using the spirit burner but with that one they didn't ask you to draw it.
I think that that's it.
Original post by Pirateprincess
We need to know how to draw the apparatus for:
Fractional distillation
simples distillation
Heat to reflux
Collecting a gas over water and in a gas syringe (for measuring rates of reaction).
Specific experiments? The electrolysis of copper chromate was on a past paper. Then there was also the determining of the enthalpy change using the spirit burner but with that one they didn't ask you to draw it.
I think that that's it.
Fractional distillation
simples distillation
Heat to reflux
Collecting a gas over water and in a gas syringe (for measuring rates of reaction).
Specific experiments? The electrolysis of copper chromate was on a past paper. Then there was also the determining of the enthalpy change using the spirit burner but with that one they didn't ask you to draw it.
I think that that's it.
Is fractional distillation the one with the Al2O3 catalyst and where 'suck-back' is a hazard?
hey im kinda stuck on a really simple thing, so if someone could kindly help me i would be grateful!
Basically if Reaction 1 has a enthalpy change of -50kjmol and Reaction 2 has an enthalpy change of -233kjmol, which reaction is more likely to occur? and could you please explain why that is. thank you!
Basically if Reaction 1 has a enthalpy change of -50kjmol and Reaction 2 has an enthalpy change of -233kjmol, which reaction is more likely to occur? and could you please explain why that is. thank you!

Original post by xstarsx67
hey im kinda stuck on a really simple thing, so if someone could kindly help me i would be grateful!
Basically if Reaction 1 has a enthalpy change of -50kjmol and Reaction 2 has an enthalpy change of -233kjmol, which reaction is more likely to occur? and could you please explain why that is. thank you!
Basically if Reaction 1 has a enthalpy change of -50kjmol and Reaction 2 has an enthalpy change of -233kjmol, which reaction is more likely to occur? and could you please explain why that is. thank you!

I think reaction 2, as it's more energetically favourable, as it is more exothermic.
Someone correct me on this if I'm wrong?
Original post by xstarsx67
hey im kinda stuck on a really simple thing, so if someone could kindly help me i would be grateful!
Basically if Reaction 1 has a enthalpy change of -50kjmol and Reaction 2 has an enthalpy change of -233kjmol, which reaction is more likely to occur? and could you please explain why that is. thank you!
Basically if Reaction 1 has a enthalpy change of -50kjmol and Reaction 2 has an enthalpy change of -233kjmol, which reaction is more likely to occur? and could you please explain why that is. thank you!

The more negative the enthalpy change the more energetically unstable a reaction is.
Any system like to be as stable as possible likes to have less energy as possible. That's a law of nature

Original post by amber206
I think reaction 2, as it's more energetically favourable, as it is more exothermic.
Someone correct me on this if I'm wrong?
Someone correct me on this if I'm wrong?
No that defiantly makes sense, thanks!
Posted from TSR Mobile
Original post by TheKingOfTSR
The more negative the enthalpy change the more energetically unstable a reaction is.
Any system like to be as stable as possible likes to have less energy as possible. That's a law of nature
Any system like to be as stable as possible likes to have less energy as possible. That's a law of nature

Oh I see, thank you for sharing your knowledge

Posted from TSR Mobile
Original post by kimmykim1
hi , in jan 2010 ques 5 i would have chosen C because in mean bond enthalpy were supposed to break all bonds but the answer is A, why is that?
The bond enthalpy you get for the reactions other than A is for 4 C-H bonds (in CH4)... you would like the mean bond enthalpy of just one C-H bond so you divide by 4
Can someone please help me with this question? January 2012 MCQ question 3:
The definition of the mole is :
A the amount of any substance which occupies a volume of 24 dm^3 at RTP
B the amount of any substance containing the same number of identical entities (??) as there are in exactly 12 g of the carbon-12 isotope.
C the number of atoms in exactly 12 g of the carbon-12 isotope
D the number of molecules in exactly 2 g of hydrogen at RTP
The correct answer is B. I don't understand why C is wrong? Can someone PLEASE explain this
The definition of the mole is :
A the amount of any substance which occupies a volume of 24 dm^3 at RTP
B the amount of any substance containing the same number of identical entities (??) as there are in exactly 12 g of the carbon-12 isotope.
C the number of atoms in exactly 12 g of the carbon-12 isotope
D the number of molecules in exactly 2 g of hydrogen at RTP
The correct answer is B. I don't understand why C is wrong? Can someone PLEASE explain this

Original post by Kurraiyo
Can someone please help me with this question? January 2012 MCQ question 3:
The definition of the mole is :
A the amount of any substance which occupies a volume of 24 dm^3 at RTP
B the amount of any substance containing the same number of identical entities (??) as there are in exactly 12 g of the carbon-12 isotope.
C the number of atoms in exactly 12 g of the carbon-12 isotope
D the number of molecules in exactly 2 g of hydrogen at RTP
The correct answer is B. I don't understand why C is wrong? Can someone PLEASE explain this
The definition of the mole is :
A the amount of any substance which occupies a volume of 24 dm^3 at RTP
B the amount of any substance containing the same number of identical entities (??) as there are in exactly 12 g of the carbon-12 isotope.
C the number of atoms in exactly 12 g of the carbon-12 isotope
D the number of molecules in exactly 2 g of hydrogen at RTP
The correct answer is B. I don't understand why C is wrong? Can someone PLEASE explain this

I don't have a clue exactly what B says either. But C tricked me also .... the number of atoms would be found by doing mole x Avogadro constant
 The number (or coefficient) doesn't represent the number of atoms - that's a really sneaky question.
The number (or coefficient) doesn't represent the number of atoms - that's a really sneaky question.Original post by kimmykim1
what is stereoisomerism ?
Stereoisomerism is when the molecules have the same molecular formula but a different three dimensional arrangement in space. It arises in molecules with restricted rotation, eg the restricted rotation about the carbon-carbon double bond in alkenes.

Original post by kimmykim1
what is stereoisomerism ?
There's 2 types... in Unit 1 you'll only need to know about Geometric isomers

They usually consist of double bonds... and the idea is that since groups can not rotate around this double bond you can get different isomers.
Here's an example:
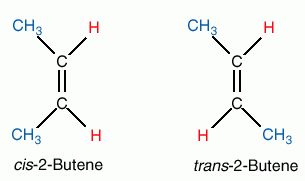
If the bond in the "middle" was a single bond... this wouldn't have been a problem since the CH3 and H can rotate around it. Here that's not the case due to the double bond.
In the image the cis trans systems has been used.... cis means that the same elements are on the same side of the double bond (hope that makes sense
 ). trans is the opposite of that as you can see.
). trans is the opposite of that as you can see. But the E Z system is much better as in some cases you will get more than 2 types of groups. In this case "E" prefix would mean that the high priority groups/elements are on opposite sides of the double bond (equivalent to trans). "Z" is the opposite..equivalent to cis.
2 "priority" groups/atoms are chosen based on their relative atomic mass... the 2 with the greatest mass will be prioritized.
Original post by Kurraiyo
Stereoisomerism is when the molecules have the same molecular formula but a different three dimensional arrangement in space. It arises in molecules with restricted rotation, eg the restricted rotation about the carbon-carbon double bond in alkenes. 

thanks

Original post by ayat94
can anybody help me with ppm what is the method to handle question like this in the exam
Well the idea with PPM (which is used for tiny amounts) is that say Xenon which makes up only 0.000009 % of the atmosphere has 0.000009 parts per 100 parts of air --> 0.000009 x 10000 = 0.09 --> 0.09 parts per million.
d you think this paper is gonna be tricky, i say that because the physics unit 1, was EXTREMELY HARD, and the biology was tricky. soo ?
Original post by ZeshanGriffin
d you think this paper is gonna be tricky, i say that because the physics unit 1, was EXTREMELY HARD, and the biology was tricky. soo ?
No point trying to figure out whether it'll be 'hard' or not.
It's about trying to do as well as you can so you're on the right side of the bell-curve, because that's how they sort out the boundaries, so try your best, and try to be as calm as possible. It'll all be done with in the morning, so try and revise a lot tonight, get a good night's sleep and you'll be fine in the morning.
 Just try your best and don't get stressed over the difficulty. Trickiness is relative, there will always be the people who complain, yet do well and didn't actually find it hard, and the people who complain regardless of the difficulty; the best thing you can do is prepare yourself. They set boundaries to the aptitude of the candidates with regards to that paper, so if it's 'tricky' but lots of people do really well anyway, the boundaries will still be medium relative to the amount of people who succeed.
Just try your best and don't get stressed over the difficulty. Trickiness is relative, there will always be the people who complain, yet do well and didn't actually find it hard, and the people who complain regardless of the difficulty; the best thing you can do is prepare yourself. They set boundaries to the aptitude of the candidates with regards to that paper, so if it's 'tricky' but lots of people do really well anyway, the boundaries will still be medium relative to the amount of people who succeed.You just have to try your best to cover all of Unit 1 as comprehensively as possible, so you're prepared with some revision.
Related discussions
- A-level Exam Discussions 2024
- GCSE Exam Discussions 2024
- ⭐niamhcheesecake's Yr 11 journal: help me conquer Gcse's :(( ⭐
- GCSE Diary
- School is killing me - Y11 "GYG" 2022
- IAL repeats cash in.
- GCSE Exam Discussions 2023
- Edexcel chemistry unit 2 mixed questions
- A Level Advice
- A Level Exam Discussions 2023
- Who will win the EFL Cup?
- Edexcel Past Papers
- Synoptic questions
- 4 weeks Until 1st Year Med Exams
- Entry requirements university of Hertfordshire
- a level chemistry
- Higher chemistry
- Chemistry 1C june 2023 exam 22nd May
- Bangor University GCSE Revision guides?
- AS/A Level Chemistry Study Group 2023/2024
Latest
Trending
Last reply 1 day ago
OCR A-LEVEL CHEMISTRY A PAPER 2 (H432/02) - 21st June [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 2 (7405/2) - 18th June 2024 [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 1 (7405/1) - 10th June 2024 [Exam Chat]Last reply 1 week ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 2 weeks ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 Higher Tier Triple (8462 2H) - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 Foundation Triple (8462 1F) - 17th May 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 5 Chem 2 Foundation - 11th June 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]Trending
Last reply 1 day ago
OCR A-LEVEL CHEMISTRY A PAPER 2 (H432/02) - 21st June [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 2 (7405/2) - 18th June 2024 [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 1 (7405/1) - 10th June 2024 [Exam Chat]Last reply 1 week ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 2 weeks ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 Higher Tier Triple (8462 2H) - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 Foundation Triple (8462 1F) - 17th May 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 5 Chem 2 Foundation - 11th June 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]




