Shapes of molecules
When a lone pair is involved in a dative covalent/ coordinate bond with another atom is it then considered as a bonding pair (when considering the shape of the moelcule?)
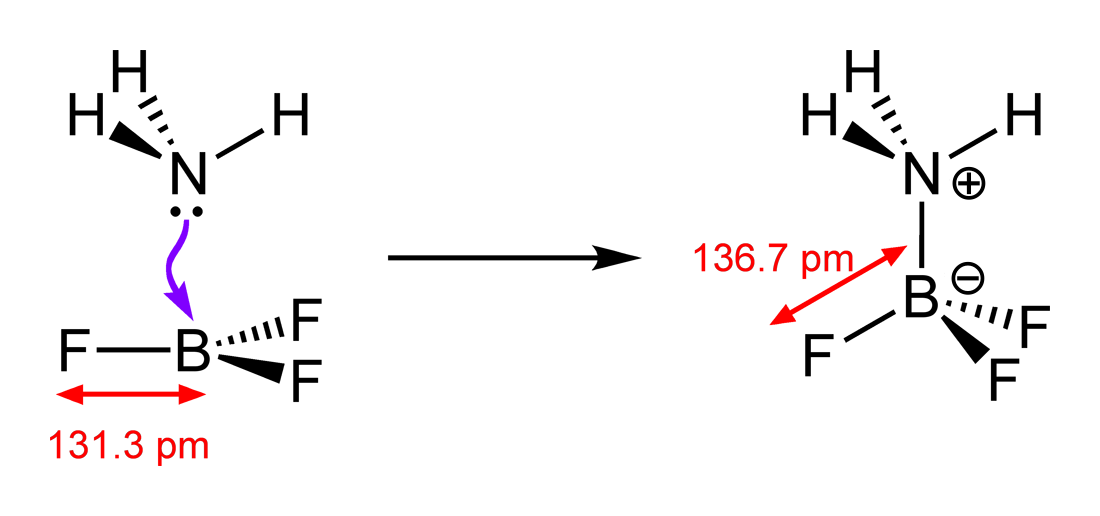
For example I thought the F-B-F angle in H3NBF3 would be 107 (trigonal) because there are 3 bonding pairs around the central B atom, and one lone pair, however it is 109.5, implying a tetrahedral shape and that the central atom has 4 bonding pairs around it.
I hope that makes sense!
For example I thought the F-B-F angle in H3NBF3 would be 107 (trigonal) because there are 3 bonding pairs around the central B atom, and one lone pair, however it is 109.5, implying a tetrahedral shape and that the central atom has 4 bonding pairs around it.
I hope that makes sense!
Original post by annaj97
When a lone pair is involved in a dative covalent/ coordinate bond with another atom is it then considered as a bonding pair (when considering the shape of the moelcule?)
For example I thought the F-B-F angle in H3NBF3 would be 107 (trigonal) because there are 3 bonding pairs around the central B atom, and one lone pair, however it is 109.5, implying a tetrahedral shape and that the central atom has 4 bonding pairs around it.
I hope that makes sense!
For example I thought the F-B-F angle in H3NBF3 would be 107 (trigonal) because there are 3 bonding pairs around the central B atom, and one lone pair, however it is 109.5, implying a tetrahedral shape and that the central atom has 4 bonding pairs around it.
I hope that makes sense!
Original post by annaj97
When a lone pair is involved in a dative covalent/ coordinate bond with another atom is it then considered as a bonding pair (when considering the shape of the moelcule?)
For example I thought the F-B-F angle in H3NBF3 would be 107 (trigonal) because there are 3 bonding pairs around the central B atom, and one lone pair, however it is 109.5, implying a tetrahedral shape and that the central atom has 4 bonding pairs around it.
I hope that makes sense!
For example I thought the F-B-F angle in H3NBF3 would be 107 (trigonal) because there are 3 bonding pairs around the central B atom, and one lone pair, however it is 109.5, implying a tetrahedral shape and that the central atom has 4 bonding pairs around it.
I hope that makes sense!
The basic shape (before VSEPR distortions) adopted by the electron domains around any atom is only dependent on the number of domains.
Four domains = tetrahedral
Original post by zetamcfc

Thank you!!
Original post by charco
The basic shape (before VSEPR distortions) adopted by the electron domains around any atom is only dependent on the number of domains.
Four domains = tetrahedral
Four domains = tetrahedral
Thank you!!
Quick Reply
Related discussions
- shape of molecules a level chem
- Shapes of mocelues
- A level chem dipole question help needed!
- a level chemistry
- a level chemistry drawing moelceules
- multiple choice Q
- Shapes of molecules
- AQA A level chemistry
- a level chemistry
- BSF polarity
- Cycloalkanes
- Chemistry - melting point
- Will the bond angle in H2O(104.5) increase if water is bind to other molecules?
- Biology Paper 2 AQA Triple Higher 2023
- Paper 3 AQA a Level biology
- a level chemistry aqa
- bonding
- NMR- drawing and labelling spectra.
- biology
- AS/A Level Chemistry Study Group 2023/2024
Latest
Trending
Last reply 1 week ago
Im confused about this chemistry question, why does it form these productsTrending
Last reply 1 week ago
Im confused about this chemistry question, why does it form these products



