Electron configuration
My class looked at this briefly so am I right in saying that whichever group is at the end of the structure is what block it is in?
For example for
1s^2 2s^2 2p^6 ….. 3d^6
Would be in group d because it’s at the end?
But an exception would be for example
1s^2 2s^2 2p^6 ….. 3d^6 4s^2
Because 4s fills before 3d, so would it still be group d?
Thanks
For example for
1s^2 2s^2 2p^6 ….. 3d^6
Would be in group d because it’s at the end?
But an exception would be for example
1s^2 2s^2 2p^6 ….. 3d^6 4s^2
Because 4s fills before 3d, so would it still be group d?
Thanks
Original post by study23!
My class looked at this briefly so am I right in saying that whichever group is at the end of the structure is what block it is in?
For example for
1s^2 2s^2 2p^6 ….. 3d^6
Would be in group d because it’s at the end?
But an exception would be for example
1s^2 2s^2 2p^6 ….. 3d^6 4s^2
Because 4s fills before 3d, so would it still be group d?
Thanks
For example for
1s^2 2s^2 2p^6 ….. 3d^6
Would be in group d because it’s at the end?
But an exception would be for example
1s^2 2s^2 2p^6 ….. 3d^6 4s^2
Because 4s fills before 3d, so would it still be group d?
Thanks
If you look at a periodic table and count through the 1s^2 2s^2 2p^6… you'll see that the "4s" elements (K and Ca) come before the "3d" elements (Sc–Zn) – 4s fills before 3d (and 5s before 4d etc). So in your 1s^2 2s^2 2p^6 ….. 3d^6 4s^2 example you'd count the 2 x 4s elements then count to the 6th 3d element, which is Fe (a d-block element). I don't think it matters which way round the 3d^6 and 4s^2 go; I tend to do s then d

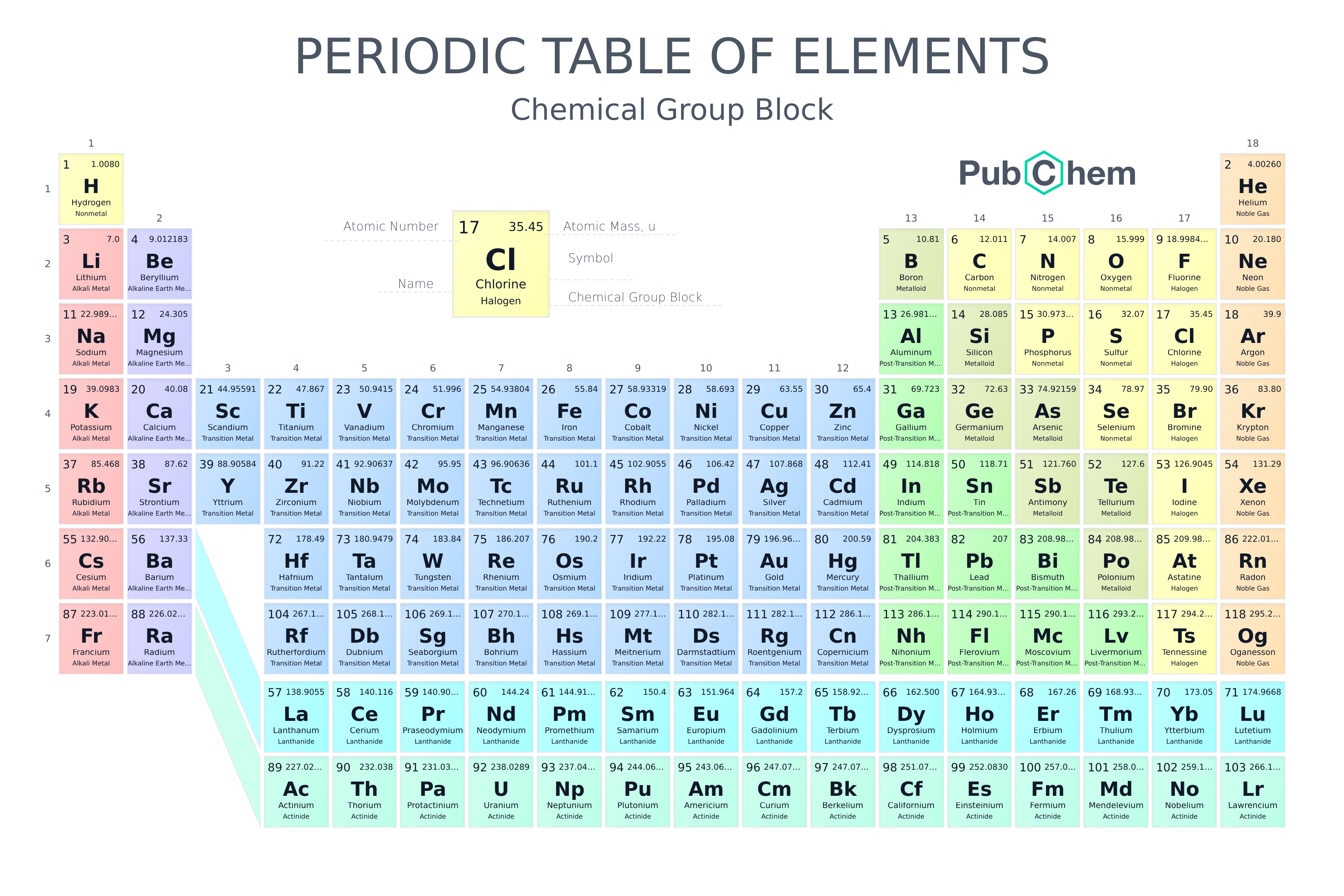
https://pubchem.ncbi.nlm.nih.gov/periodic-table/
I find it easiest to count (not necessarily one by one, just through the blocks until I get to the one of interest
 )
)Original post by bl0bf1sh
If you look at a periodic table and count through the 1s^2 2s^2 2p^6… you'll see that the "4s" elements (K and Ca) come before the "3d" elements (Sc–Zn) – 4s fills before 3d (and 5s before 4d etc). So in your 1s^2 2s^2 2p^6 ….. 3d^6 4s^2 example you'd count the 2 x 4s elements then count to the 6th 3d element, which is Fe (a d-block element). I don't think it matters which way round the 3d^6 and 4s^2 go; I tend to do s then d 

https://pubchem.ncbi.nlm.nih.gov/periodic-table/
I find it easiest to count (not necessarily one by one, just through the blocks until I get to the one of interest )
)


https://pubchem.ncbi.nlm.nih.gov/periodic-table/
I find it easiest to count (not necessarily one by one, just through the blocks until I get to the one of interest
 )
)Thanks 😊
Quick Reply
Related discussions
- NF3 lewis structure
- Question about periodic table
- Chemistry electron configuration help
- Chemistry AS level Question (electronic configuration
- a level chemistry
- chemistry multiple choice
- Electronic Configurations
- Why does Scandium only exist in the 3+ ion
- Periodic table: Period 3 group 7
- Does the period number also help with electron arrangement?
- year 12 a level chem question
- Electron configuration of Co(II) ion??
- Chemistry electronegativity
- AS CHEMISTRY ionisation energy multiple choice question help?
- Chemistry Alevel question
- A level chemistry past paper question help
- Chem as help!
- Why doesnt Fe2SO4 exist in nature?
- Organic Chemistry help
- Chemistry Questions
Latest
Trending
Last reply 1 week ago
Im confused about this chemistry question, why does it form these productsTrending
Last reply 1 week ago
Im confused about this chemistry question, why does it form these products



