AQA Physics PHYA5 - Thursday 18th June 2015 [Exam Discussion Thread]
Scroll to see replies
When they say that it has 0.375 times as many carbon-14 atoms as an equal mass of living wood do they mean its comparing the content of carbon-14 in modern day wood to old wood from the boat??? Not sure what that statement means in the picture attached.
Also, the question the final temperature of the cola drink. In the mark scheme it subtracts 30 from the final temeprature for the change in temperature of the beaker which is initially at 30 degrees celcius but shouldn't be final temperature - initial temperature???
Thanks in advance for any help.
Posted from TSR Mobile
Also, the question the final temperature of the cola drink. In the mark scheme it subtracts 30 from the final temeprature for the change in temperature of the beaker which is initially at 30 degrees celcius but shouldn't be final temperature - initial temperature???
Thanks in advance for any help.

Posted from TSR Mobile
Original post by CD223
How do you know the volume remains unchanged? It only says the temperature does.
Posted from TSR Mobile
Posted from TSR Mobile
I was mistakenly considering the tank and the pump seperately and the volume of the tank doesn't change so that's where I got confused
Original post by Sbarron
I was mistakenly considering the tank and the pump seperately and the volume of the tank doesn't change so that's where I got confused
Oh I see! Yeah when they mean volume in the equations they're referring to the volume of the gas itself, so when you're given multiple volumes just think of what volume the gas will occupy and use that in the formulas

Posted from TSR Mobile
Original post by MSB47
When they say that it has 0.375 times as many carbon-14 atoms as an equal mass of living wood do they mean its comparing the content of carbon-14 in modern day wood to old wood from the boat??? Not sure what that statement means in the picture attached.
Also, the question the final temperature of the cola drink. In the mark scheme it subtracts 30 from the final temeprature for the change in temperature of the beaker which is initially at 30 degrees celcius but shouldn't be final temperature - initial temperature???
Thanks in advance for any help.
Posted from TSR Mobile
Also, the question the final temperature of the cola drink. In the mark scheme it subtracts 30 from the final temeprature for the change in temperature of the beaker which is initially at 30 degrees celcius but shouldn't be final temperature - initial temperature???
Thanks in advance for any help.

Posted from TSR Mobile
The way I do those temperature ones and decide wether to take initial from final or visa versa is that if there is energy lost you want your temperature value to be negative... Works for me every time

With the boat one, if I remember correctly they are comparing the number of c14 in the boat (a dead tree) to the C14 in a living tree
Original post by CD223
Oh I see! Yeah when they mean volume in the equations they're referring to the volume of the gas itself, so when you're given multiple volumes just think of what volume the gas will occupy and use that in the formulas 
Posted from TSR Mobile

Posted from TSR Mobile
Yep ok my memory of that was getting a bit rusty so thanks for freshening it up! LolThe whole valve part didn't help
Original post by MSB47
When they say that it has 0.375 times as many carbon-14 atoms as an equal mass of living wood do they mean its comparing the content of carbon-14 in modern day wood to old wood from the boat??? Not sure what that statement means in the picture attached.
Also, the question the final temperature of the cola drink. In the mark scheme it subtracts 30 from the final temeprature for the change in temperature of the beaker which is initially at 30 degrees celcius but shouldn't be final temperature - initial temperature???
Thanks in advance for any help.
Posted from TSR Mobile
Also, the question the final temperature of the cola drink. In the mark scheme it subtracts 30 from the final temeprature for the change in temperature of the beaker which is initially at 30 degrees celcius but shouldn't be final temperature - initial temperature???
Thanks in advance for any help.

Posted from TSR Mobile
The Carbon 14 content of once living organisms gradually decays over time. As the ratio is less than one, it may help to think of it as "the ancient piece of wood has 0.375 times the content of the piece of living wood" which makes sense - the living wood should have more as its alive and photosynthesising. Whereas the ancient piece of wood is dead and therefore has a small fraction of the Carbon 14 content of the living piece of wood.
The other question refers to when the cola and glass are in "thermal equilibrium". In other words they are the same temperature so there is no net transfer of thermal energy between them.
The cola is initially at 3 degrees and the glass is at 30 degrees.
Logically, the cola will warm up due to the heating effect from the glass so there will be a net transfer of thermal energy to the cola from the glass until it reaches thermal equilibrium. Equally, the glass will cool as it transfers energy to the cola.
This means the glass cools from 30 degrees to Tf, and the cola warms up from 3 degrees to Tf.
If you place the Tf terms so it reads (final temperature - initial temperature) for both then the thermal energy of the glass will be negative, which leads to the incorrect answer.
The reason for this is because the mark scheme equates the loss of energy from the glass to the gain in energy of the cola. This makes both quantities positive and allows the final temperature to be calculated.
Posted from TSR Mobile
Original post by CD223
The Carbon 14 content of once living organisms gradually decays over time. As the ratio is less than one, it may help to think of it as "the ancient piece of wood has 0.375 times the content of the piece of living wood" which makes sense - the living wood should have more as its alive and photosynthesising. Whereas the ancient piece of wood is dead and therefore has a small fraction of the Carbon 14 content of the living piece of wood.
The other question refers to when the cola and glass are in "thermal equilibrium". In other words they are the same temperature so there is no net transfer of thermal energy between them.
The cola is initially at 3 degrees and the glass is at 30 degrees.
Logically, the cola will warm up due to the heating effect from the glass so there will be a net transfer of thermal energy to the cola from the glass until it reaches thermal equilibrium. Equally, the glass will cool as it transfers energy to the cola.
This means the glass cools from 30 degrees to Tf, and the cola warms up from 3 degrees to Tf.
If you place the Tf terms so it reads (final temperature - initial temperature) for both then the thermal energy of the glass will be negative, which leads to the incorrect answer.
The reason for this is because the mark scheme equates the loss of energy from the glass to the gain in energy of the cola. This makes both quantities positive and allows the final temperature to be calculated.
Posted from TSR Mobile
The other question refers to when the cola and glass are in "thermal equilibrium". In other words they are the same temperature so there is no net transfer of thermal energy between them.
The cola is initially at 3 degrees and the glass is at 30 degrees.
Logically, the cola will warm up due to the heating effect from the glass so there will be a net transfer of thermal energy to the cola from the glass until it reaches thermal equilibrium. Equally, the glass will cool as it transfers energy to the cola.
This means the glass cools from 30 degrees to Tf, and the cola warms up from 3 degrees to Tf.
If you place the Tf terms so it reads (final temperature - initial temperature) for both then the thermal energy of the glass will be negative, which leads to the incorrect answer.
The reason for this is because the mark scheme equates the loss of energy from the glass to the gain in energy of the cola. This makes both quantities positive and allows the final temperature to be calculated.
Posted from TSR Mobile
Ahh ok I get the first part now, thanks.
For the second part because one is losing thermal energy and one is gaining thermal energy can you equate the two equations like this...
mc-(dT)=mc(dT)
where dT= change in temperature and because the glass is cooling it temperature drops hence a negative change of temperature and then that can deduce the (30-Tf) rather than (Tf-30)??
Original post by MSB47
Ahh ok I get the first part now, thanks.
For the second part because one is losing thermal energy and one is gaining thermal energy can you equate the two equations like this...
mc-(dT)=mc(dT)
where dT= change in temperature and because the glass is cooling it temperature drops hence a negative change of temperature and then that can deduce the (30-Tf) rather than (Tf-30)??
For the second part because one is losing thermal energy and one is gaining thermal energy can you equate the two equations like this...
mc-(dT)=mc(dT)
where dT= change in temperature and because the glass is cooling it temperature drops hence a negative change of temperature and then that can deduce the (30-Tf) rather than (Tf-30)??
Yeah - if you try it the other way around you get an answer like -6.8 degrees which doesn't make sense either!
Posted from TSR Mobile
Original post by gcsestuff
How do I know when to use I1/i2 = (X1/x2 )^2
And when to use i1/i2=(x2/X1) ^2
I'm so confused
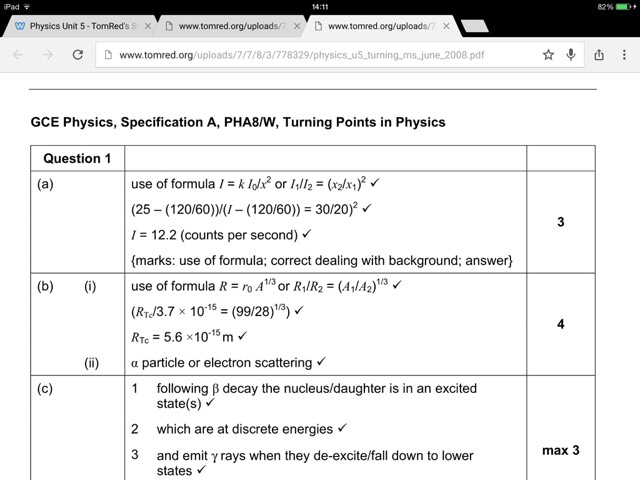
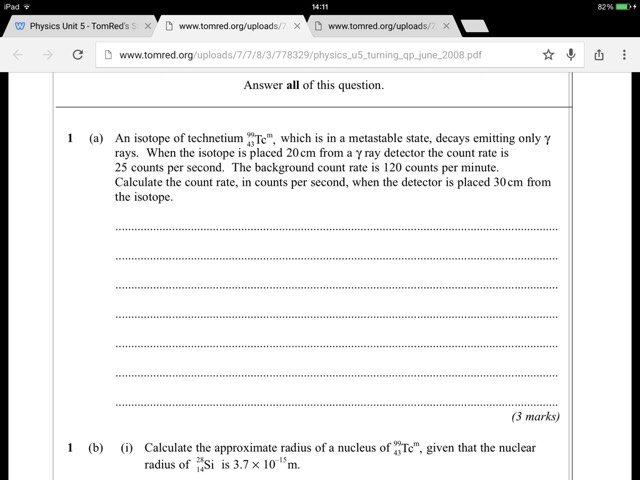
Posted from TSR Mobile
And when to use i1/i2=(x2/X1) ^2
I'm so confused

Posted from TSR Mobile
This is how I've done it
Original post by MSB47
When they say that it has 0.375 times as many carbon-14 atoms as an equal mass of living wood do they mean its comparing the content of carbon-14 in modern day wood to old wood from the boat??? Not sure what that statement means in the picture attached.
Also, the question the final temperature of the cola drink. In the mark scheme it subtracts 30 from the final temeprature for the change in temperature of the beaker which is initially at 30 degrees celcius but shouldn't be final temperature - initial temperature???
Thanks in advance for any help.
Posted from TSR Mobile
Also, the question the final temperature of the cola drink. In the mark scheme it subtracts 30 from the final temeprature for the change in temperature of the beaker which is initially at 30 degrees celcius but shouldn't be final temperature - initial temperature???
Thanks in advance for any help.

Posted from TSR Mobile
Here is a similar type of question and what I've done to find the mass of ice
Original post by betbi3etwerrd
I would've thought that they provided us with r_0 value?
I had one question where I was expected to work it out first and then put it in my further calculations and it defiantly was not 1.4*10^15 ... That gave the wrong answer
(edited 8 years ago)
Does anyone know where the 1.22 comes from in this formula for working out the radius with diffraction? And do we need to remember the formula for the exam?
For turning points I think it would be a good idea to compile a list of "significance of results".
E.g. The significant of Einstein's photon model of light is that light can be shows wave-particle duality: Light's particle behavior is photoelectric effect and wave behavior is diffraction/interference.
Please correct my one if it's wrong and post similar ones for things like Hertz, Millikan, Thomson, Michelson-Morley, Maxwell, etc.
E.g. The significant of Einstein's photon model of light is that light can be shows wave-particle duality: Light's particle behavior is photoelectric effect and wave behavior is diffraction/interference.
Please correct my one if it's wrong and post similar ones for things like Hertz, Millikan, Thomson, Michelson-Morley, Maxwell, etc.
Original post by Sbarron
Thanks that question seems pretty decent!
Original post by Sbarron
I had one question where I was expected to work it out first and then put it in my further calculations and it defiantly was not 1.4*10^15 ... That gave the wrong answer
Was that June 2012? I think I remember that question.
Posted from TSR Mobile
Anyone know if there is a difference between heat energy and thermal energy? Which is better to use?
Original post by CD223
Yeah - if you try it the other way around you get an answer like -6.8 degrees which doesn't make sense either!
Posted from TSR Mobile
Posted from TSR Mobile
Thanks for the help

Original post by Sbarron
This is how I've done it

Thanks I understand it now
 done loads of questions on inverse square law as its got to come up ! Just got to make sure I use common sense. Eg it moves further away it gets less intense
done loads of questions on inverse square law as its got to come up ! Just got to make sure I use common sense. Eg it moves further away it gets less intense Posted from TSR Mobile
Original post by slaven123
Does anyone know where the 1.22 comes from in this formula for working out the radius with diffraction? And do we need to remember the formula for the exam?
I don't believe we need to know the derivation but yes, you do need to know that the first minimum appears where
Or equally,
Where R is the nuclear radius and D is the nuclear diameter.
Posted from TSR Mobile
Original post by MSB47
Thanks for the help 

No problem! Thanks for flagging it up - hopefully we don't make that mistake on Thursday now!
Posted from TSR Mobile
Quick Reply
Related discussions
- A-level Exam Discussions 2024
- GCSE Exam Discussions 2024
- A Level Exam Discussions 2023
- AQA A-Level Chemistry Paper 2 (7405/2) - 18th June 2024 [Exam Chat]
- GCSE Exam Discussions 2023
- OCR B A-level Physics Paper 2 Advancing Physics (H557/02) - 9th June 2023 [Exam Chat]
- AQA GCSE Physics Paper 2 (Foundation Combined) 8464/2F - 16th June 2023 [Exam Chat]
- AQA GCSE Physical sciences (Higher Combined) 8465/4H - 13th June 2023 [Exam Chat]
- OCR A-LEVEL CHEMISTRY A PAPER 2 (H432/02) - 21st June [Exam Chat]
- OCR A A-level Physics Paper 1 Modelling Physics (H556/01) - 24th May 2024 [Exam Chat]
- OCR B A-level Physics Paper 3 Advancing Physics (H557/03) - 15th Jun 2023 [Exam Chat]
- AQA A Level Physical Education Paper 1 7582/1 - 26 May 2022 [Exam Chat]
- GCSE exam advice
- AQA GCSE Food Preparation and Nutrition Paper 1 (8585/W) - 19th June 2024 [Exam Chat]
- AQA GCSE Geography Paper 2 (8035/2) - 5th June 2024 [Exam Chat]
- AQA GCSE Geography Paper 3 (8035/3) - 14th June 2024 [Exam Chat]
- GCSE 2024 timetable
- AQA GCSE Chemistry Paper 2 Foundation Triple (8462 2F) - 11th June 2024 [Exam Chat]
- English Lit Paper 2 leak??!?!!
- AQA GCSE Food Preparation and Nutrition - Tuesday 19th June 2024 [Exam Chat]
Latest
Trending
Last reply 17 hours ago
AQA A-level Physics Paper 1 (7408/1) - 24th May 2024 [Exam Chat]Last reply 2 days ago
AQA GCSE Physics Paper 1 (Higher Tier triple) 8463/1H - 22nd May 2024 [Exam Chat]Last reply 1 week ago
AQA GCSE Physics Paper 1 (Higher Combined) 8464/1H - 22nd May 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Physics Paper 2 (Higher Tier triple) 8463/2H - 14th June 2024 [Exam Chat]Last reply 3 weeks ago
OCR A A-level Physics Paper 1 Modelling Physics (H556/01) - 24th May 2024 [Exam Chat]Last reply 1 month ago
AQA A-level Physics Paper 2 (7408/2) - 9th June 2023 [Exam Chat]Last reply 2 months ago
Edexcel A Level Physics Paper 3: 9PH0 03 - 15th June 2023 [Exam Chat]Last reply 3 months ago
AQA A-level Physics Paper 1 (7408/1) - 24th May 2023 [Exam Chat]Physics Exams
1190
Last reply 4 months ago
Edexcel GCSE Physics Paper 1 Higher Combined 1SC0 1PH - 25th May 2023 [Exam Chat]Last reply 5 months ago
Edexcel GCSE Physics Paper 1 Higher Tier Triple 1PH0 1H - 25th May 2023 [Exam Chat]Last reply 5 months ago
AQA A-level Physics Paper 3 (7408/3) - 15th June 2023 [Exam Chat]Last reply 10 months ago
Edexcel GCSE Physics Paper 2 Higher Tier Triple 1PH0 2H - 16th June 2023 [Exam Chat]Last reply 10 months ago
OCR B A-level Physics Paper 3 Advancing Physics (H557/03) - 15th Jun 2023 [Exam Chat]Last reply 10 months ago
OCR GCSE Physics A Paper 4 Higher Tier (J249/04) - 16th June 2023 [Exam Chat]Trending
Last reply 17 hours ago
AQA A-level Physics Paper 1 (7408/1) - 24th May 2024 [Exam Chat]Last reply 2 days ago
AQA GCSE Physics Paper 1 (Higher Tier triple) 8463/1H - 22nd May 2024 [Exam Chat]Last reply 1 week ago
AQA GCSE Physics Paper 1 (Higher Combined) 8464/1H - 22nd May 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Physics Paper 2 (Higher Tier triple) 8463/2H - 14th June 2024 [Exam Chat]Last reply 3 weeks ago
OCR A A-level Physics Paper 1 Modelling Physics (H556/01) - 24th May 2024 [Exam Chat]Last reply 1 month ago
AQA A-level Physics Paper 2 (7408/2) - 9th June 2023 [Exam Chat]Last reply 2 months ago
Edexcel A Level Physics Paper 3: 9PH0 03 - 15th June 2023 [Exam Chat]Last reply 3 months ago
AQA A-level Physics Paper 1 (7408/1) - 24th May 2023 [Exam Chat]Physics Exams
1190
Last reply 4 months ago
Edexcel GCSE Physics Paper 1 Higher Combined 1SC0 1PH - 25th May 2023 [Exam Chat]Last reply 5 months ago
Edexcel GCSE Physics Paper 1 Higher Tier Triple 1PH0 1H - 25th May 2023 [Exam Chat]Last reply 5 months ago
AQA A-level Physics Paper 3 (7408/3) - 15th June 2023 [Exam Chat]Last reply 10 months ago
Edexcel GCSE Physics Paper 2 Higher Tier Triple 1PH0 2H - 16th June 2023 [Exam Chat]Last reply 10 months ago
OCR B A-level Physics Paper 3 Advancing Physics (H557/03) - 15th Jun 2023 [Exam Chat]Last reply 10 months ago
OCR GCSE Physics A Paper 4 Higher Tier (J249/04) - 16th June 2023 [Exam Chat]



