sn2 mechanism for making propan-1-ol from OH- and 1-bromopropane?
Can anyone outline the mechanism steps or show them please?
Original post by thebrahmabull
Can anyone outline the mechanism steps or show them please?
Backside attack of the brominated carbon by the hydroxide nucleophile, with a transition state in which both Br and OH are bonded to the carbon. Bromide is eliminated.
Nucleophilic substitution
Nucleophile OH: attacks carbon, due to slightly positive charge (Curly arrow from lone pair to carbon),
C-Br bond's pair "Jumps" onto the bromine breaking the bond to form Br: thus leaving propan-1-ol (Curly arrow from C-Br bond to Br atom).
Conditions: Under reflux
Nucleophile OH: attacks carbon, due to slightly positive charge (Curly arrow from lone pair to carbon),
C-Br bond's pair "Jumps" onto the bromine breaking the bond to form Br: thus leaving propan-1-ol (Curly arrow from C-Br bond to Br atom).
Conditions: Under reflux
Original post by RME11
Nucleophilic substitution
Nucleophile OH: attacks carbon, due to slightly positive charge (Curly arrow from lone pair to carbon),
C-Br bond's pair "Jumps" onto the bromine breaking the bond to form Br: thus leaving propan-1-ol (Curly arrow from C-Br bond to Br atom).
Conditions: Under reflux
Nucleophile OH: attacks carbon, due to slightly positive charge (Curly arrow from lone pair to carbon),
C-Br bond's pair "Jumps" onto the bromine breaking the bond to form Br: thus leaving propan-1-ol (Curly arrow from C-Br bond to Br atom).
Conditions: Under reflux
Hydroxide, not a hydroxy radical and Bromide, not a Bromine radical. Charges are important.
Remember that there's also inversion of the tetrahedral transition state geometry if they want you to show that.
(edited 8 years ago)
Original post by alow
Hydroxide, not a hydroxy radical and Bromide, not a Bromine radical. Charges are important.
Let's not nitpick.
That is what I was insinuating - If it was a hydroxy radical they would only be a single unpaired electron, not a lone pair as shown.
Original post by Plantagenet Crown
Remember that there's also inversion of stereochemistry if they want you to show that.
Not here, but generally yes.
Original post by Plantagenet Crown
Remember that there's also inversion of stereochemistry if they want you to show that.
1-bromopropane is not chiral ...
Original post by RME11
Let's not nitpick.
That is what I was insinuating - If it was a hydroxy radical they would only be a single unpaired electron, not a lone pair as shown.
That is what I was insinuating - If it was a hydroxy radical they would only be a single unpaired electron, not a lone pair as shown.
Being exact is important. You would rightfully be deducted marks for not indicating charge.
Original post by thebrahmabull
Can anyone outline the mechanism steps or show them please?
Original post by alow
Backside attack of the brominated carbon by the hydroxide nucleophile, with a transition state in which both Br and OH are bonded to the carbon. Bromide is eliminated.
Original post by RME11
Nucleophilic substitution
Nucleophile OH: attacks carbon, due to slightly positive charge (Curly arrow from lone pair to carbon),
C-Br bond's pair "Jumps" onto the bromine breaking the bond to form Br: thus leaving propan-1-ol (Curly arrow from C-Br bond to Br atom).
Conditions: Under reflux
Nucleophile OH: attacks carbon, due to slightly positive charge (Curly arrow from lone pair to carbon),
C-Br bond's pair "Jumps" onto the bromine breaking the bond to form Br: thus leaving propan-1-ol (Curly arrow from C-Br bond to Br atom).
Conditions: Under reflux
Original post by Plantagenet Crown
Remember that there's also inversion of the tetrahedral transition state geometry if they want you to show that.
Original post by Infraspecies
Not here, but generally yes.
Original post by charco
1-bromopropane is not chiral ...
Thanks guys! I did a mechanism here
 is it correct?
is it correct?Original post by thebrahmabull
Thanks guys! I did a mechanism here  is it correct?
is it correct?
 is it correct?
is it correct?It's not incorrect, but here's some tips for good practice:
-Get used to writing bonds as dashes and wedges
-When drawing a transition state, write it in square brackets with a sort of hash tag at the top right (where the charge normally would be for a complex ion)
-This is a bit nitpicky, but when drawing the TS make sure it makes a Y shape - in your drawing, the H's are completely trans to each other and the C2H5 is cis to both, which could be interpreted wrong (your lack of dashes and wedges don't help)
Here's an illustration:
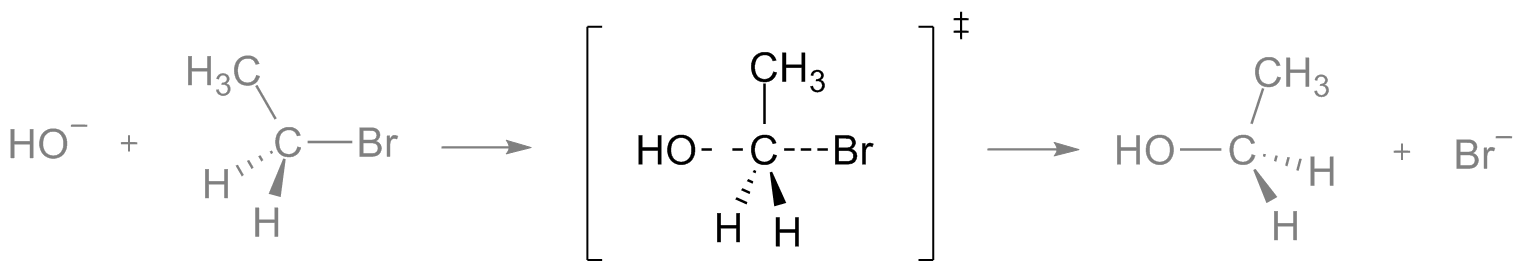
Original post by InadequateJusticex
It's not incorrect, but here's some tips for good practice:
-Get used to writing bonds as dashes and wedges
-When drawing a transition state, write it in square brackets with a sort of hash tag at the top right (where the charge normally would be for a complex ion)
-This is a bit nitpicky, but when drawing the TS make sure it makes a Y shape - in your drawing, the H's are completely trans to each other and the C2H5 is cis to both, which could be interpreted wrong (your lack of dashes and wedges don't help)
Here's an illustration:
-Get used to writing bonds as dashes and wedges
-When drawing a transition state, write it in square brackets with a sort of hash tag at the top right (where the charge normally would be for a complex ion)
-This is a bit nitpicky, but when drawing the TS make sure it makes a Y shape - in your drawing, the H's are completely trans to each other and the C2H5 is cis to both, which could be interpreted wrong (your lack of dashes and wedges don't help)
Here's an illustration:

thanks! may I know the significance of the hash like sign outside the square bracket?
Original post by thebrahmabull
thanks! may I know the significance of the hash like sign outside the square bracket?
It's just a symbol/shorthand to say that it's a transition state

Quick Reply
Related discussions
- Which is correct?
- Kc Titration Question
- The eqm constant question
- is but-1-ene the same as butene
- Esterification
- Edexcel A-Level Chem Paper 2 Advanced Organic and Physical Chemistry [Exam Chat]
- AS Organic Chem Question
- enthalpy change of combustion alchols
- SN1 and SN2 reactions (chemistry)
- Help chemistry
- SN2 nucleophilic substitution -OH
- Atom economy
- Hess’s law
- AS chemistry paper 2 2022 AQ
- The suffix en for alkenes
- Chem alevel help
- A level chemistry optical isomerism MC questions
- Organic chemistry learning a-level
- A-level Chemistry Study Group 2022-2023
- propan-1-ol and propanal
Latest
Trending
Last reply 1 week ago
Im confused about this chemistry question, why does it form these productsTrending
Last reply 1 week ago
Im confused about this chemistry question, why does it form these products



