Scroll to see replies
Original post by HiMyNameIsRiley
please can someone help with the attached questions
(OCR A A-level chemistry)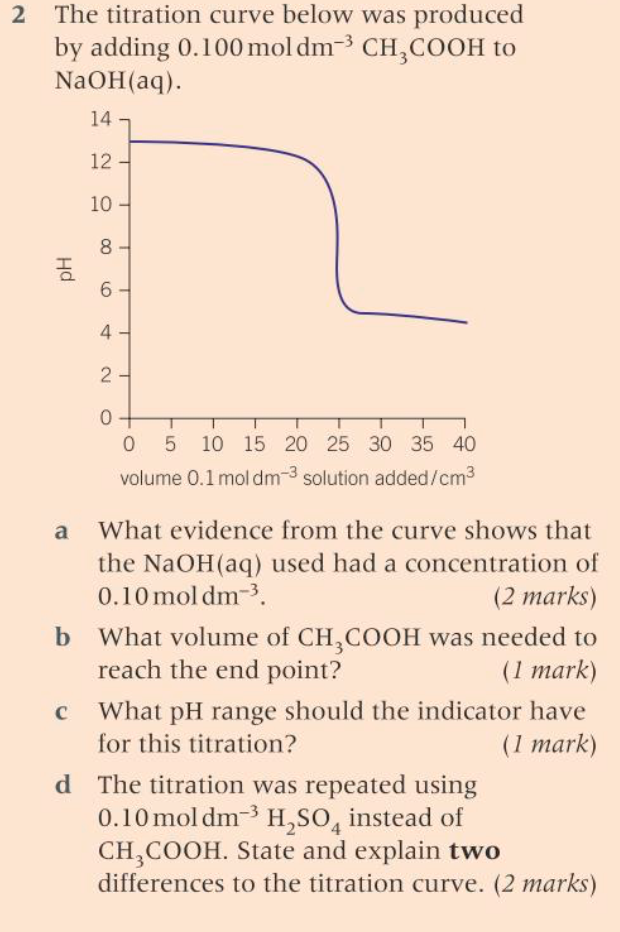
(OCR A A-level chemistry)
Okay, for part (a) what role does the concentration of a base play? Hint: what does the concentration of an acid determine?
Original post by ilovephysmath
Okay, for part (a) what role does the concentration of a base play? Hint: what does the concentration of an acid determine?
does the concentration determine how much the reagent will dissociate?
Original post by HiMyNameIsRiley
does the concentration determine how much the reagent will dissociate?
Nope. pH, remember?
Original post by ilovephysmath
Nope. pH, remember?
oh yeah, so if the concentration of NaOH is 0.100 mol dm-3 does this mean pOH is -log(0.1) = 1 ?
Original post by HiMyNameIsRiley
oh yeah, so if the concentration of NaOH is 0.100 mol dm-3 does this mean pOH is -log(0.1) = 1 ?
Yes. And then...?
(edited 3 years ago)
Original post by ilovephysmath
Oh yeah sorry my bad, yes pOH is 1
ok, and since pH=14-pOH, pH would be 13? and on the graph the line goes to 13; pH tends to 13, indicating that the conc. of NaOH is 0.100 mol dm-3. Is that correct?
Original post by HiMyNameIsRiley
ok, and since pH=14-pOH, pH would be 13? and on the graph the line goes to 13; pH tends to 13, indicating that the conc. of NaOH is 0.100 mol dm-3. Is that correct?
Bingo! There you go

Okay, now for part b. How do you think we’re supposed to approach the question? Where is the ‘endpoint’?
Original post by ilovephysmath
Bingo! There you go 

yay!! thanks
Original post by ilovephysmath
Okay, now for part b. How do you think we’re supposed to approach the question? Where is the ‘endpoint’?
i think the end point is the vertical line? so when the pH is between 6-10 ?
Original post by HiMyNameIsRiley
yay!! thanks
i think the end point is the vertical line? so when the pH is between 6-10 ?
i think the end point is the vertical line? so when the pH is between 6-10 ?
Yes, the endpoint lies halfway up the vertical line. What will you do next?
Original post by ilovephysmath
Yes, the endpoint lies halfway up the vertical line. What will you do next?
like draw a line from that point downwards to the x-axis?
would the volume of CH3COOH be 25cm3 ?
Original post by HiMyNameIsRiley
like draw a line from that point downwards to the x-axis?
would the volume of CH3COOH be 25cm3 ?
would the volume of CH3COOH be 25cm3 ?
Yes!
Original post by HiMyNameIsRiley
like draw a line from that point downwards to the x-axis?
would the volume of CH3COOH be 25cm3 ?
would the volume of CH3COOH be 25cm3 ?
Okay part c now. Do you know what boxes an indicator should tick to be suitable for a titration??
Original post by ilovephysmath
Okay part c now. Do you know what boxes an indicator should tick to be suitable for a titration??
the indicator has to have a colour change in the same pH range as the vertical line section of the curve?
Original post by HiMyNameIsRiley
the indicator has to have a colour change in the same pH range as the vertical line section of the curve?
Yes, and you can use your data booklet to find that indicator
Original post by HiMyNameIsRiley
the indicator has to have a colour change in the same pH range as the vertical line section of the curve?
Part d. There are two ways h2so4 is different from ch3cooh. What do you think those two ways are?
Original post by ilovephysmath
Part d. There are two ways h2so4 is different from ch3cooh. What do you think those two ways are?
-its a strong acid, CH3COOH is a weak acid
-so therefore it will fully dissociate, unlike CH3COOH
Original post by HiMyNameIsRiley
-its a strong acid, CH3COOH is a weak acid
-so therefore it will fully dissociate, unlike CH3COOH
-so therefore it will fully dissociate, unlike CH3COOH
Yes, and? The second point is just an elaboration of the first. Hint: the first dissociation of H2SO4 is complete.....
another hint: H2SO4 is diprotic. How will that affect the titration curve?
Quick Reply
Related discussions
- TSR Study Together - STEM vs Humanities!
- chemistry or psychology a level??
- A level chemistry for a biology degree? (help please!!)
- A Level Choices Help (Aqa Physics V Chemistry)
- pls be my chemistry tutor !!
- Chemistry A-level
- Chemistry or physics A level
- What university has the lowest GCSE requirements for medicine?
- I'm unsure whether I should do chemistry A levels
- Is it worth doing chemistry for a neuroscience degree?
- Is there anyway to do extra chemistry outside of school?
- A-level combo for Pharmacy
- Worried about degree difficulty - Chemistry
- Can I go into biochem or work in the medical field with these alevels?
- How to make my application strong
- Biomed vs Biochem vs chemistry
- Chemistry A level
- AS/A Level Chemistry Study Group 2023/2024
- Biology a level
- Biomed / Pharmacy
Latest
Trending
Last reply 1 week ago
Im confused about this chemistry question, why does it form these productsTrending
Last reply 1 week ago
Im confused about this chemistry question, why does it form these products



