Intermolecular forces!
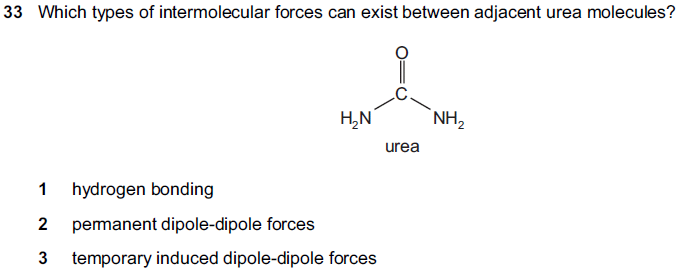
I thought that there will only be hydrogen bonds, and permanent dipole-permanent dipole forces. But as mark scheme says that all the options are correct, so is this the explanation that no matter what there will always be instantaneous dipole- induced dipole forces between any molecules?

all three, yes.
basically, you always have no. 3 whatever the molecule.
if there is a permanent dipole, you will have 2 in addition.
if there is a hydrogen bonded to an electronegative atom, you will also get 1 in addition
basically, you always have no. 3 whatever the molecule.
if there is a permanent dipole, you will have 2 in addition.
if there is a hydrogen bonded to an electronegative atom, you will also get 1 in addition
yup, no matter what there will always be instantaneous dipole- induced dipole forces between any molecules. you have just answered your own question, dear sir 

sorry, is there an echo in here?!


Original post by Farseer
Hydrogen ions in the gas phase won't have instantaneous dipole - induced dipole forces 

which is why I said molecule (as did both my co-workers).
(edited 12 years ago)
Original post by Plato's Trousers
which is why I said molecule (as did both my co-workers).
plato, plato....i like the echo "praise"

you've got followers, i see...
Quick Reply
Related discussions
- gcse macromolecules
- Bond breaking and intermolecular force A level
- Boiling point of halogenoalkanes
- Using your knowledge of chemistry
- Hydrogen bond a level chem
- Chemistry HW help
- Bonding A level chem - AQA
- Learndirect- presentation advice
- Polymer q
- AS Chemistry Multiple choice question help TQ
- Chemistry question dipole
- Electrostatic forces
- Intermolecular Forces
- dipole dipole vs vanderwaals forces difference
- Bonding
- chemical bonding
- van der waals
- What is the difference between simple molecular and simple covalent substances?
- Struggling with A Level Chemistry…
- Higher chemistry
Latest
Trending
Last reply 1 week ago
Im confused about this chemistry question, why does it form these productsTrending
Last reply 1 week ago
Im confused about this chemistry question, why does it form these products

