This discussion is now closed.
Check out other Related discussions
- SQA Higher Chemistry - Paper 1 Multiple Choice - 23rd May 2024 [Exam Chat]
- ⭐niamhcheesecake's Yr 11 journal: help me conquer Gcse's :(( ⭐
- Should I apply to Cambridge?
- A Level Chemistry (OCR B Salters Chemistry)
- AS/A Level Chemistry Study Group 2023/2024
- A-level Exam Discussions 2024
- School is killing me - Y11 "GYG" 2022
- chemistry
- GCSE Diary
- SQA Higher Mathematics - Paper 1 Non-calculator - 13th May 2024 [Exam Chat]
- gyg gcse 2024!
- Analysis of Advanced Notice Article for Chemistry B Salters
- As level exam in 10 days, need help
- 🙈Last minute A level Motivation
- Revision website for OCR B (salters) chemistry
- My GCSE journey!! 🦞
- Politics or religious studies for A-level? Urgent!!
- OCR Chemistry B (Salters) - Anybody doing this?
- SQA Higher French - Reading and Directed Writing - 26th April 2024 [Exam Chat]
- SQA Higher Human Biology - Paper 1 Multiple Choice - 15th May 2024 [Exam Chat]
F334 (19/06/2013 9:00AM) - Salters Chemistry (Chemistry of Materials) Revision Thread
Scroll to see replies
Original post by CureSam
18.2cm^3=0.0182dm^3
0.0182*0.02=3.6*10^-4 moles of MnO4 (vol*conc=moles)
3.6*10^-4*(5/2)=9*10^-4 (moles of H2O2 from the equation)
That number of H2O2 is in 10cm^3, which is 0.01dm^3, giving a concentration of 0.09moles/dm^3, but the solution has been diluted by 10, meaning the original concentration 0.9mol/dm^3.
Hope this helps!
0.0182*0.02=3.6*10^-4 moles of MnO4 (vol*conc=moles)
3.6*10^-4*(5/2)=9*10^-4 (moles of H2O2 from the equation)
That number of H2O2 is in 10cm^3, which is 0.01dm^3, giving a concentration of 0.09moles/dm^3, but the solution has been diluted by 10, meaning the original concentration 0.9mol/dm^3.
Hope this helps!
Thank you

Original post by tasniaa
how's everyone feeling about this exam?
Much better than the first time I sat it, now that i've done F334 and F335.
Original post by suncake
https://docs.google.com/file/d/1sdHMYjo9qqZslsPAlbdN_DoQTxO0gEJapvUKPDHMPMnjkPKS3egQH2XDf5ja/edit?usp=sharing
Here you go. Yeah, ignore the incorrect scribbles then And excuse my awful scanning skills
And excuse my awful scanning skills 
Here you go. Yeah, ignore the incorrect scribbles then
 And excuse my awful scanning skills
And excuse my awful scanning skills 
Brilliant, thanks for uploading. The paper actually didn't seem too bad, just those god-awful grade boundaries that put my two grades below where I needed.
Could you explain 2(c)(iii)? I don't understand why ethanoic acid can hydrogen bond, but compound D only has pd-pd bonding.
Thanks loads if you can.

Original post by tsr1
C14H12O3 + 3OH- ----> (C14H9O3) 3- + 3H2O
can someone please explain why we get C14H9O3) 3- ?
can someone please explain why we get C14H9O3) 3- ?
3 OH groups that can donate 3 protons/hydrogen atoms to the base (OH-), so you're left with 3 negative oxygen atoms, giving a minus charge of 3.
Original post by abzy1234
3 OH groups that can donate 3 protons/hydrogen atoms to the base (OH-), so you're left with 3 negative oxygen atoms, giving a minus charge of 3.
3 OH on the alcohol will donate the H+ isn't? and thanks it was just in an equation form which made it harder for me to understand..

Original post by tsr1
3 OH on the alcohol will donate the H+ isn't? and thanks it was just in an equation form which made it harder for me to understand..

Yup and no problem

Original post by tsr1
3 OH on the alcohol will donate the H+ isn't? and thanks it was just in an equation form which made it harder for me to understand..

Be careful, a hydroxyl group on an alcohol won't donate a proton, only a cardboxylic acid.
Original post by CureSam
You must have got like 90% ums on that?
Nope
 74/90 raw marks was 76/90 ums, so about 84% ums.
74/90 raw marks was 76/90 ums, so about 84% ums.Original post by gingernat
Brilliant, thanks for uploading. The paper actually didn't seem too bad, just those god-awful grade boundaries that put my two grades below where I needed.
Could you explain 2(c)(iii)? I don't understand why ethanoic acid can hydrogen bond, but compound D only has pd-pd bonding.
Thanks loads if you can.
Could you explain 2(c)(iii)? I don't understand why ethanoic acid can hydrogen bond, but compound D only has pd-pd bonding.
Thanks loads if you can.

No bother

Carboxylic acids can form hydrogen bonds with each other because of the OH in the COOH group.
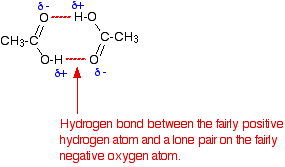
Esters like compound D however do not contain an OH group, so don't have a suitable slightly positive hydrogen that can bond to the slightly negative oxygen on their carbonyl group. The hydrogens on the CH bond can't form H bonds because C and H have similar electronegativities, so it's not a polar bond and the H isn't slightly negative.
I hope that made sense (and is actually correct
 )... I hate intermolecular bonds
)... I hate intermolecular bonds 
Posted from TSR Mobile
Original post by CureSam
Be careful, a hydroxyl group on an alcohol won't donate a proton, only a cardboxylic acid.
u make sense but the question said its an alcohol... confused
How do we calculate the rate of disappearance? Last question on Jan13
Now for F334.
Posted from TSR Mobile
Posted from TSR Mobile
Original post by tasniaa
Today's paper was good practice for mass spec

Posted from TSR Mobile
Original post by tasniaa
This paper is the bane of my life
Got AAA in the AS units
an A(*) in A2 coursework, a mid B in Chem of Design
all the above being first attempts
yet a E then D then C in chem of mateirals
resulting in a B overall

Original post by 11hokj1
does anyone have the Jan 2013 MARK SCHEME? 

Here you go

Original post by super121
Here you go 

Thank you. And good luck next week!

Original post by abzy1234
I'm literally sick with all the chemistry revision. Constantly regurgitating stuff one learnt from last year certainly ain't fun 

Same here. And I absolutely hate F334. Thought it was harder than F335! But yesterday's F335 was horrible too

Posted from TSR Mobile
Original post by 11hokj1
Same here. And I absolutely hate F334. Thought it was harder than F335! But yesterday's F335 was horrible too 
Posted from TSR Mobile

Posted from TSR Mobile
I know right! F335 has badly demotivated me for the F334 exam, knowing that the former has the heavier weighting doesn't help either. Well I guess all we can do is aim to do the best for this exam and hope we get the grade we want

Related discussions
- SQA Higher Chemistry - Paper 1 Multiple Choice - 23rd May 2024 [Exam Chat]
- ⭐niamhcheesecake's Yr 11 journal: help me conquer Gcse's :(( ⭐
- Should I apply to Cambridge?
- A Level Chemistry (OCR B Salters Chemistry)
- AS/A Level Chemistry Study Group 2023/2024
- A-level Exam Discussions 2024
- School is killing me - Y11 "GYG" 2022
- chemistry
- GCSE Diary
- SQA Higher Mathematics - Paper 1 Non-calculator - 13th May 2024 [Exam Chat]
- gyg gcse 2024!
- Analysis of Advanced Notice Article for Chemistry B Salters
- As level exam in 10 days, need help
- 🙈Last minute A level Motivation
- Revision website for OCR B (salters) chemistry
- My GCSE journey!! 🦞
- Politics or religious studies for A-level? Urgent!!
- OCR Chemistry B (Salters) - Anybody doing this?
- SQA Higher French - Reading and Directed Writing - 26th April 2024 [Exam Chat]
- SQA Higher Human Biology - Paper 1 Multiple Choice - 15th May 2024 [Exam Chat]
Latest
Trending
Last reply 2 days ago
OCR A-LEVEL CHEMISTRY A PAPER 2 (H432/02) - 21st June [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 2 (7405/2) - 18th June 2024 [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 1 (7405/1) - 10th June 2024 [Exam Chat]Last reply 2 weeks ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 2 weeks ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 Higher Tier Triple (8462 2H) - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 Foundation Triple (8462 1F) - 17th May 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 5 Chem 2 Foundation - 11th June 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]Trending
Last reply 2 days ago
OCR A-LEVEL CHEMISTRY A PAPER 2 (H432/02) - 21st June [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 2 (7405/2) - 18th June 2024 [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 1 (7405/1) - 10th June 2024 [Exam Chat]Last reply 2 weeks ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 2 weeks ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 Higher Tier Triple (8462 2H) - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 Foundation Triple (8462 1F) - 17th May 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 5 Chem 2 Foundation - 11th June 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]



