Equilibria, Energetics and Elements (F325) - June 2011 Exam.
Scroll to see replies
Original post by J DOT A
The answer is 30% guys.
We have HCl twice.... so we need to take away one from the other.
We have HCl twice.... so we need to take away one from the other.
can u plz show ur working
Is the more negative electrode potential the one that is being oxidised? Which side are the electrons on when writing the equation?
Original post by INeedToRevise
Is the more negative electrode potential the one that is being oxidised? Which side are the electrons on when writing the equation?
When writing the overall equation electrons should be on either side and should cancel as one species is being oxidised and the other is being reduced. The more negative electrode potential is the one which is oxidised, yes.

hey guys! i was just wondering how you get your results for OCR chem on results day? i live in northern ireland and we get our CCEA results through the internet via a pin number from midnight on results day - do OCR have anything like that?
thanks
thanks

Original post by INeedToRevise
Is the more negative electrode potential the one that is being oxidised? Which side are the electrons on when writing the equation?
Yes that's correct, the electrons flow from the negative to the positive.
I think the book says that by convention you show the electrons on the left!
Original post by tripodd
Guys, I get 0.013 instead of 0.13 as the MS says on the Specimen paper for question 2(d) - does anyone get this too? think it's an error
i got 0.13 when i did this paper, but typing everything into my calculator again im getting 0.013? dunno, specimen papers are always crap
Need help on this question please 
"a student prepared 2 solutions : Solution A made by mixing together 25cm3 0.010mol dm3 aqueous NaOH with 50vm3 0.010 mol dm3 CH3COOH. Solution A is a buffer solution.
Solution B by mixing together 25cm3 0.020mol dm3 NaOH with 50cm3 0.010mol dm3
CH3COOH. Solution B not a buffer solution"
Explain why solution A is a buffer and solution B is not.
Anyone?

"a student prepared 2 solutions : Solution A made by mixing together 25cm3 0.010mol dm3 aqueous NaOH with 50vm3 0.010 mol dm3 CH3COOH. Solution A is a buffer solution.
Solution B by mixing together 25cm3 0.020mol dm3 NaOH with 50cm3 0.010mol dm3
CH3COOH. Solution B not a buffer solution"
Explain why solution A is a buffer and solution B is not.
Anyone?
Oh just one more:
"You got 0.100moldm3 NaOH and 0.100moldm3 CH3COOH (pH 2.9). 50cm3 of 0.100moldm3 NaOH is gradually added to 25cm3 of 0.100mol dm3 CH3COOH.
Sketch the titration curve"
How would i go about doing that :|
(These both questions are from unifying concepts - jan '05 )
Thanks in advance x
"You got 0.100moldm3 NaOH and 0.100moldm3 CH3COOH (pH 2.9). 50cm3 of 0.100moldm3 NaOH is gradually added to 25cm3 of 0.100mol dm3 CH3COOH.
Sketch the titration curve"
How would i go about doing that :|
(These both questions are from unifying concepts - jan '05 )

Thanks in advance x
Original post by Ecstacy.
Need help on this question please 
"a student prepared 2 solutions : Solution A made by mixing together 25cm3 0.010mol dm3 aqueous NaOH with 50vm3 0.010 mol dm3 CH3COOH. Solution A is a buffer solution.
Solution B by mixing together 25cm3 0.020mol dm3 NaOH with 50cm3 0.010mol dm3
CH3COOH. Solution B not a buffer solution"
Explain why solution A is a buffer and solution B is not.
Anyone?

"a student prepared 2 solutions : Solution A made by mixing together 25cm3 0.010mol dm3 aqueous NaOH with 50vm3 0.010 mol dm3 CH3COOH. Solution A is a buffer solution.
Solution B by mixing together 25cm3 0.020mol dm3 NaOH with 50cm3 0.010mol dm3
CH3COOH. Solution B not a buffer solution"
Explain why solution A is a buffer and solution B is not.
Anyone?
in solution A the weak acid is in excess, so you get some weak acid remaining, but then NaOH + CH3COOH ->CH3COONa + H2O
and then the CH3COONa --> dissciates to CH3COO- so you have weak acid and its conjugate base.
in B, the amounts of moles of NaOH and CH3COOH are equal so all the CH3COOH reacts with all the NaOH to create salt and water, no acid remains.
Original post by M_I
Can some one explain this please?
Its June 2002 - Unifying Concepts - Question 4)b)i)
Question and MS attatched.
I don't get how it works.


Thanks.
Its June 2002 - Unifying Concepts - Question 4)b)i)
Question and MS attatched.
I don't get how it works.

Thanks.

The mark scheme covers it mostly, however you had to realise that citric acid would dissociate in H20, as it is an acid:
So H3A -> 3H+ + A3^-
In solution NaHCO3 dissociates itself to form Na+ + HCO3^-
H+ from the citric acid reacts with the carbonate to form: H20 + CO2
H+ + HCO3- -> H20 + CO2
Original post by Ecstacy.
Oh just one more:
"You got 0.100moldm3 NaOH and 0.100moldm3 CH3COOH (pH 2.9). 50cm3 of 0.100moldm3 NaOH is gradually added to 25cm3 of 0.100mol dm3 CH3COOH.
Sketch the titration curve"
How would i go about doing that :|
(These both questions are from unifying concepts - jan '05 )
Thanks in advance x
"You got 0.100moldm3 NaOH and 0.100moldm3 CH3COOH (pH 2.9). 50cm3 of 0.100moldm3 NaOH is gradually added to 25cm3 of 0.100mol dm3 CH3COOH.
Sketch the titration curve"
How would i go about doing that :|
(These both questions are from unifying concepts - jan '05 )

Thanks in advance x
Hey your general curve is going to look like this:
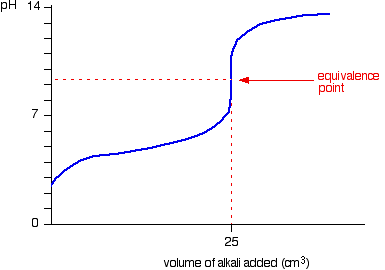
Then you need to take the volumes into account, concentrations are the same so the equivalence point will be at 25 cm3 and the mid point will be at 9 (as the strong base dominates) your starting pH is 2.39 and you ending pH will be close to 14.

Can anyone please help me with this question? It was on the Jan 09 UC paper.
"Compound E has the formula CxHyOz. 4.362g of E was burned in oxygen to form 5.119g of CO2 and 1.575g H2O. Mass spec showed a peak at 150.
Calculate the molecular formula of E."
I had a look at the mark scheme but I can't follow what it's doing :s Do you think we'll get something similar in our paper?
"Compound E has the formula CxHyOz. 4.362g of E was burned in oxygen to form 5.119g of CO2 and 1.575g H2O. Mass spec showed a peak at 150.
Calculate the molecular formula of E."
I had a look at the mark scheme but I can't follow what it's doing :s Do you think we'll get something similar in our paper?
Original post by student777
Original post by student777
Can anyone please help me with this question? It was on the Jan 09 UC paper.
"Compound E has the formula CxHyOz. 4.362g of E was burned in oxygen to form 5.119g of CO2 and 1.575g H2O. Mass spec showed a peak at 150.
Calculate the molecular formula of E."
I had a look at the mark scheme but I can't follow what it's doing :s Do you think we'll get something similar in our paper?
"Compound E has the formula CxHyOz. 4.362g of E was burned in oxygen to form 5.119g of CO2 and 1.575g H2O. Mass spec showed a peak at 150.
Calculate the molecular formula of E."
I had a look at the mark scheme but I can't follow what it's doing :s Do you think we'll get something similar in our paper?
I'd hope not! It has no relevance to our spec whatsoever!
Original post by sc0307
I'd hope not! It has no relevance to our spec whatsoever!
You say that, but they do ask similar calculation questions! The June 2010 paper had a question where you have to find the formula of a chromium thing, so I was asking just in case something like it comes up...

Original post by student777
Can anyone please help me with this question? It was on the Jan 09 UC paper.
"Compound E has the formula CxHyOz. 4.362g of E was burned in oxygen to form 5.119g of CO2 and 1.575g H2O. Mass spec showed a peak at 150.
Calculate the molecular formula of E."
I had a look at the mark scheme but I can't follow what it's doing :s Do you think we'll get something similar in our paper?
"Compound E has the formula CxHyOz. 4.362g of E was burned in oxygen to form 5.119g of CO2 and 1.575g H2O. Mass spec showed a peak at 150.
Calculate the molecular formula of E."
I had a look at the mark scheme but I can't follow what it's doing :s Do you think we'll get something similar in our paper?
Right ok so lets look at the key facts here:
5.119 = CO2 1.575 = H2O
You know that CO2 Mr = 44 and in it C is 12
so you can work out a fraction of C in 5.119 of CO2 which is 12/44 x 5.119 = 1.396
Same with H2O = 18 and H is 2
2/18 x 1.575 = 0.175
Now you have the g of H2 and C, so you can subtract that from the total to find O2
4.362 - (1.396 + 0.175) = 2.791
Now you can work out the empirical firmula
C: H: O
1.396: 0.175: 2.791
divide through by their Mr
C 1.396/12 = 0.1163
H 0.175/1 = 0.175
O 2.791/16 = 0.1744
0.1163:0.175:0.1744
simplifying it
1: 1.5: 1.5
to get a whole number ratio you can multiply by 2 to get
2: 3: 3
This gives you C2H3O3 to get 75
150/75 = 2
2 x (C2H3O3) = C4H6O6
Hope this helps

Original post by sportycricketer
Right ok so lets look at the key facts here:
5.119 = CO2 1.575 = H2O
You know that CO2 Mr = 44 and in it C is 12
so you can work out a fraction of C in 5.119 of CO2 which is 12/44 x 5.119 = 1.396
Same with H2O = 18 and H is 2
2/18 x 1.575 = 0.175
Now you have the g of H2 and C, so you can subtract that from the total to find O2
4.362 - (1.396 + 0.175) = 2.791
Now you can work out the empirical firmula
C: H: O
1.396: 0.175: 2.791
divide through by their Mr
C 1.396/12 = 0.1163
H 0.175/1 = 0.175
O 2.791/16 = 0.1744
0.1163:0.175:0.1744
simplifying it
1: 1.5: 1.5
to get a whole number ratio you can multiply by 2 to get
2: 3: 3
This gives you C2H3O3 to get 75
150/75 = 2
2 x (C2H3O3) = C4H6O6
Hope this helps
5.119 = CO2 1.575 = H2O
You know that CO2 Mr = 44 and in it C is 12
so you can work out a fraction of C in 5.119 of CO2 which is 12/44 x 5.119 = 1.396
Same with H2O = 18 and H is 2
2/18 x 1.575 = 0.175
Now you have the g of H2 and C, so you can subtract that from the total to find O2
4.362 - (1.396 + 0.175) = 2.791
Now you can work out the empirical firmula
C: H: O
1.396: 0.175: 2.791
divide through by their Mr
C 1.396/12 = 0.1163
H 0.175/1 = 0.175
O 2.791/16 = 0.1744
0.1163:0.175:0.1744
simplifying it
1: 1.5: 1.5
to get a whole number ratio you can multiply by 2 to get
2: 3: 3
This gives you C2H3O3 to get 75
150/75 = 2
2 x (C2H3O3) = C4H6O6
Hope this helps

Thank you! It makes sense now

Quick Reply
Related discussions
- A-level Exam Discussions 2024
- Grade Growth Chronicles | From C's to A's (23-24)
- How can I get a 9 in GCSE history?
- Over 500 questions on AQA Bio Unit 4 + Current Spec and old Spec papers + MS!
- My attempt at not sabotaging my future career!
- GCSE Exam Discussions 2024
- Day after masturbation
- Which exam boards offering biology Alevel do NOT have a practical exam?
- Edexcel IGCSE Chemistry | PAPER 1
- Audio files for GCSE French Listening??
- AQA Chem Unit 1 May 22nd 2015 *OFFICIAL THREAD*
- As level chemistry
- AQA Environmental science paper 2
- Energetics chem question
- Chemistry paper 2 igcse edexcel
- Canada vs UK
- PSYB4 JUN11 mark scheme?
- OCR A-Level Chemistry B Paper 3 (H433/03) - 23rd June 2023 [Exam Chat]
- chemistry as level equilibria help
- Edexcel Past Papers
Latest
Trending
Last reply 1 day ago
OCR A-LEVEL CHEMISTRY A PAPER 2 (H432/02) - 21st June [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 2 (7405/2) - 18th June 2024 [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 1 (7405/1) - 10th June 2024 [Exam Chat]Last reply 1 week ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 2 weeks ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 Higher Tier Triple (8462 2H) - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 Foundation Triple (8462 1F) - 17th May 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 5 Chem 2 Foundation - 11th June 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]Trending
Last reply 1 day ago
OCR A-LEVEL CHEMISTRY A PAPER 2 (H432/02) - 21st June [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 2 (7405/2) - 18th June 2024 [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 1 (7405/1) - 10th June 2024 [Exam Chat]Last reply 1 week ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 2 weeks ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 Higher Tier Triple (8462 2H) - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 Foundation Triple (8462 1F) - 17th May 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 5 Chem 2 Foundation - 11th June 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]



