Chemistry A level Question
Suggest why the addition of anhydrous magnesium chloride to water resultsin an increase in temperature?
The markscheme says that the answer is" the bonds created between the Mg2+ and cl- ion release energy as bond making is exothermic"
I dont understand as I thought when ionic compounds dissolve they are pulled apart due to the electrostatic attractions between the polar water molecules and the ions. Why are they bonding together again?
Thanks
The markscheme says that the answer is" the bonds created between the Mg2+ and cl- ion release energy as bond making is exothermic"
I dont understand as I thought when ionic compounds dissolve they are pulled apart due to the electrostatic attractions between the polar water molecules and the ions. Why are they bonding together again?
Thanks

any ideas?
When Magnesium Chloride dissolves in water, the Mg2+ and Cl- ions dissociate in solution, of course energy is required to break the lattice structure. However, an excess of energy is in fact released from the ionic bond making of water molecules surrounding the Mg2+ and Cl- ions, so-called 'clustering' of water molecules. Overall, more energy is released from the process of dissolving/bond making, this means the reaction is exothermic. Hence an increase in temperature.
This image shows a similar situation when Sodium chloride dissolves, notice the partial negative and partial positive charges of a water molecule.
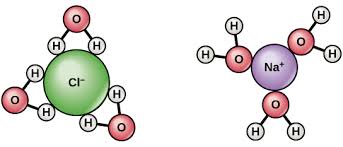
This image shows a similar situation when Sodium chloride dissolves, notice the partial negative and partial positive charges of a water molecule.
Original post by RDB1826
When Magnesium Chloride dissolves in water, the Mg2+ and Cl- ions dissociate in solution, of course energy is required to break the lattice structure. However, an excess of energy is in fact released from the ionic bond making of water molecules surrounding the Mg2+ and Cl- ions, so-called 'clustering' of water molecules. Overall, more energy is released from the process of dissolving/bond making, this means the reaction is exothermic. Hence an increase in temperature.
This image shows a similar situation when Sodium chloride dissolves, notice the partial negative and partial positive charges of a water molecule.

This image shows a similar situation when Sodium chloride dissolves, notice the partial negative and partial positive charges of a water molecule.
Thank you!
Quick Reply
Related discussions
- TSR Study Together - STEM vs Humanities!
- GCSE Exam Discussions 2024
- Question Banks
- Revision for A level
- Chemistry A-level
- Required practicals chemistry A level
- AS/A Level Chemistry Study Group 2023/2024
- Which A-level is the hardest and why?
- Pharmacy interview
- Are the CGP books good for these A level subjects?
- Undergrad Oxford Chemistry interview concerns
- Should I do Chemistry or Biology A-level?
- How to get A/A* in A level chemistry
- OCR Chemistry B (Salters) - Anybody doing this?
- Ocr chemistry a exam-style questions
- Biomedical Science
- which science is the hardest? (chemistry, physics or biology)
- Physics for Oxford Chemistry?
- Chemistry or English literature A-levels
- Struggling with A Level Chemistry…
Latest
Trending
Last reply 1 day ago
Edexcel A Level Politics Paper 1 (9PL0 01) - 21st May 2024 [Exam Chat]A-levels
10
Trending
Last reply 1 day ago
Edexcel A Level Politics Paper 1 (9PL0 01) - 21st May 2024 [Exam Chat]A-levels
10




