Original post by Medavies
Hi, I was just wondering if I could have some help. I recently missed a lesson due to illness and we went over why we use red in a colorimetry practical when using a blue copper sulphate solution. Any help would be great???
Thank you!!
Thank you!!
The solution appears blue because it transmits blue light and absorbs the other wavelengths. The colours absorbed by a solution is called the complementary colour. There are charts to show you the complementary colour of solutions.
https://en.wikipedia.org/wiki/Complementary_colors#/media/File:RGB_color_wheel.svg
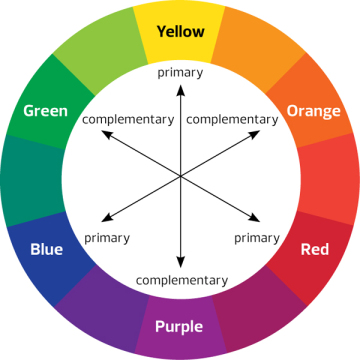
If a solution appears blue, then it absorbs in the red/orange region of the spectrum. To increase the sensitivity of the colorimeter the filter only provides light of a complementary colour.
Quick Reply
Related discussions
- BTEC applied science unit 19
- Entry requirements university of Hertfordshire
- Universities
- Can u get into MPharm without Chemistry?
- Veterinary medicine with BTEC
- University MPharm Entry Requirements
- Can i do radiographer degree with health and social care btect level 3
- Medicine with a BTEC?
- Can 1st-year BTEC Applied Science qualify for Pharmacy at uni?
- Btec applied science l3 to pharmacy
- Getting into uni with Btecs
- BTEC Level 3 in applied science
- BTEC level 3 Applied Science to pharmacy
- What subjects do you need for a radiography degree?
- will i be able to do biomedicine??? ;(
- i want to do pharmacy but only have 3 gcses
- not sure if ill get accepted into pharmacy
- BTEC - General Chat Thread
- Pharmacist
- a levels/diploma
Latest
Trending
Last reply 1 week ago
Im confused about this chemistry question, why does it form these productsTrending
Last reply 1 week ago
Im confused about this chemistry question, why does it form these products



